Unwin and Deeming 2019 report,
yet again, the hypothesis of pterosaur hatchling flight. They add this time, “the application of four contrasting quantitative approaches allows a more precise identification of the developmental status of embryos revealing, for the first time to our knowledge, the presence of middle and late developmental stages as well as individuals that were at term.”
Earlier
here, here and here middle, late and term developments were published online.
The authors add this time,
“We also identify a predicted relationship between egg size and shape and the developmental stage of embryos contained within. Small elongate eggs contain embryos at an earlier stage of development than larger rounder eggs which contain more fully developed embryos.”
Earlier
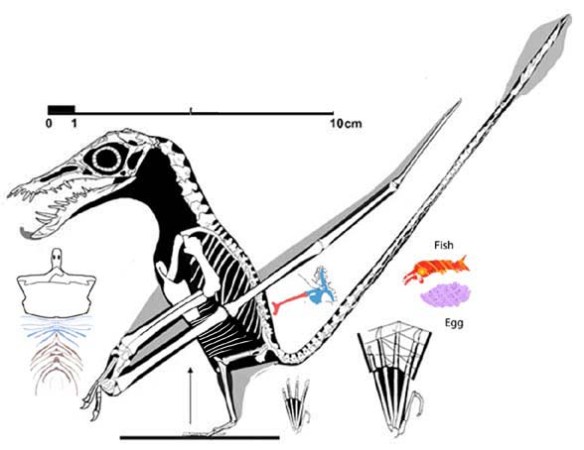
here, here and here, egg shape was matched to pterosaur rostrum length with the ctenochasmatid embryo, Pterodaustro (Figs. 3, 4) having the longer egg and the IVPP V13758 anurognathid (Figs. 1, 2) embryo having the rounder egg. It is also worthwhile to consider the alternate hypothesis presented earlier here for the varied sizes and shapes for Hamipterus eggs in a lepidosaur context (see below).
The authors add this time,
“Early ossification of the vertebral column, limb girdles and principal limb bones involved some heterochronic shifts in appearance times, most notably of manus digit IV, and facilitated full development of the flight apparatus prior to hatching.”
This has been known
since the first appearance of the IVPP anurognathid embryo (Figs. 1, 2) in 2004 and reported nearly immediately thereafter by Unwin and Deeming. That’s really stretching out a single idea over more than a decade.

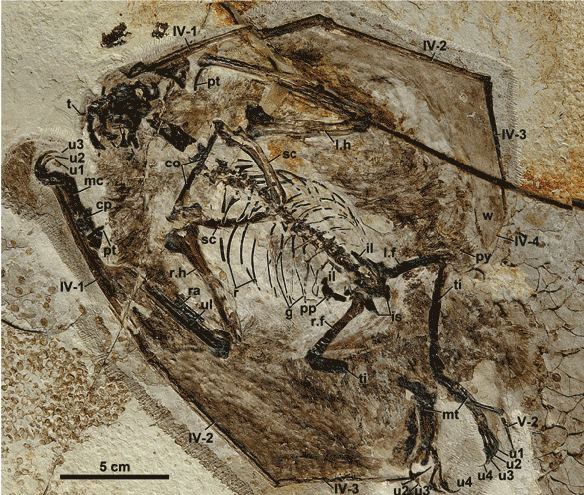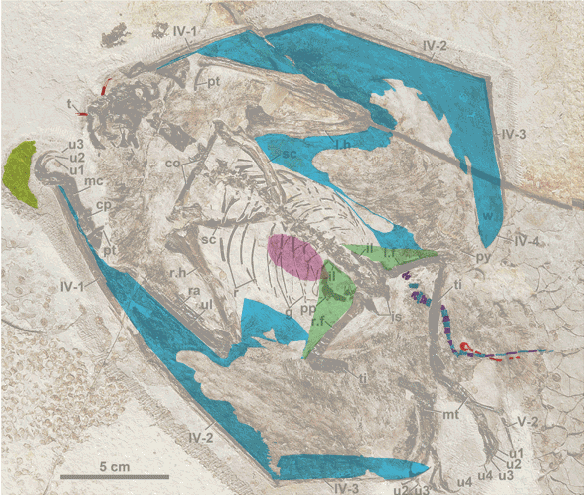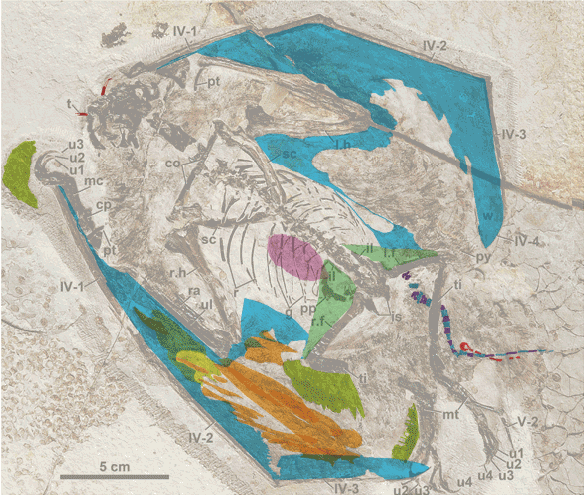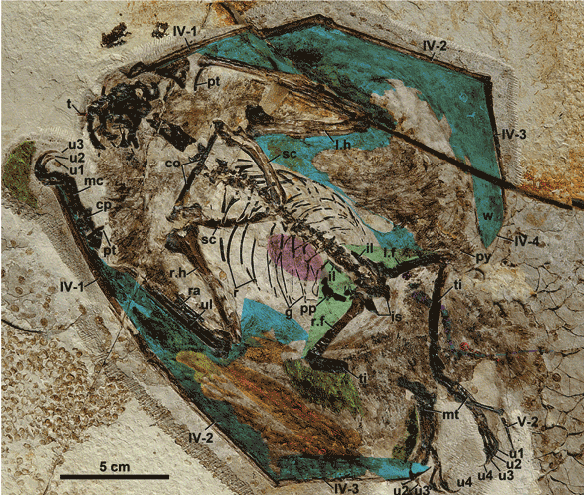
Figure 1. Click to enlarge. A magnitude of more detail was gleaned from this fossil (the IVPP egg/embryo) using the DGS method.
Over the last two decades, workers must have vowed
not to touch any lepidosaur/fenestrasaur hypotheses for fear of confirming an amateur’s findings. Nor have they added tiny pterosaurs to phylogenetic analyses.
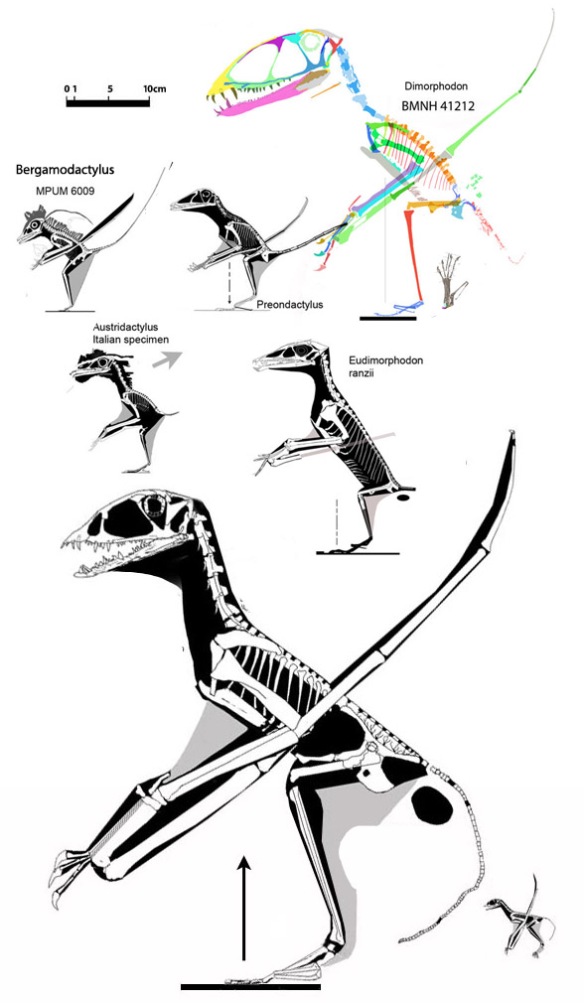
Figure 2. The IVPP embryo anurognathid (lower right) compared to other basal pterosaurs, including an adult IVPP embryo, 8x larger.
Unwin and Deeming follow the invalidated hypothesis
that pterosaurs were archosaurs that laid and buried their eggs at an early stage of development, much as birds and crocs do. Not only do workers openly admit they lack pterosaur precursors within Archosauria, birds and crocs follow an allometric growth trajectory after hatching with a short snout and large eyes.

Figure x. Added a few hours after publication. Figure from Unwin and Deeming 2019. Apparently these authors saw fewer details in these pterosaur eggs than were present. Compare to figures 1 and 3. No reconstructions were attempted by Unwin and Deeming, so they didn’t realize the embryos had adult proportions (Fig. 2) or could be scored in a phylogenetic analysis. So much potential. So little study.
On the other hand,
pterosaurs, like other lepidosaurs, follow an isometric growth series (Figs. 4, 5). as documented most clearly by Zhejiangopterus online here and Pterodaustro here, but also in a large Rhamphorhynchus juvenile here (‘the Vienna specimen’ and see citation below).

Figure 3. Original interpretations (2 frames black/white) vs. new interpretation using DGS (color). Note: the premaxilla is in the lower right corner. The back of the skull is in the upper right corner. And see figure 4 for a growth series.
In the SuppData, the authors report,
“While most recent studies have concluded that pterosaurs belong within Ornithodira [S3, S4] some analyses have located them outside this clade, although still within Archosauromorpha [S5, S6]. Here, we accept the majority view, that pterosaurs are ornithodirans, with an extant phylogenetic bracket consisting of crocodiles and birds.”
You might think they cited Peters 2000
as their ‘minority view.’ If so, you would be wrong. No citation to Peters 2000 was mentioned. S5 is Bennett 1996 where he nested pterosaurs tentatively with Scleromochlus. That was invalidated by Peters 2000 who simply added several taxa to Bennett’s published matrix of taxa and characters, and those of three other workers. S6 is Bennett 2012/13, where Bennett also ignores taxa proposed by Peters 2000, 2007.
If you’ll recall,
Bennett 2012/2013 reports that pterosaurs nested between the lumbering and aquatic archosauriforms Proterosuchus and Erythrosuchus. That moves the nesting away from Scleromochlus, proterochampsids and parasuchians, the previous archosaur ‘favorite candidates for most pterosaur workers. I shudder when I peek into their minds.
Thus Bennett’s curse,
“You will not be published, and if you are published, you will not be cited,” comes true once again. And now you know, once again, why I chose online publishing after seven or so academic publications. Not sure if not having a PhD is the issue, or if criticizing the hypotheses of PhDs is the real problem.

Figure 4. The V263 specimen compared to other Pterodaustro specimens to scale.
Readers need no reminding that phylogenetic analyses by Peters 2000
that tested prior pterosaur outgroup candidates has been enhanced online since 2011 with a steady stream of additional taxa. In that study pterosaurs nest with taxa identified by Peters 2000, and those taxa nest with taxa identified by Peters 2007, among them, the lepidosaur, Huehuecuetzpalli, all ignored by Unwin and Deeming. (You can download the Tritosauria paper here.)

Figure 5. Click to enlarge. There are several specimens of Zhejiangopterus. The two pictured in figure 2 are the two smallest above at left. Also shown is a hypothetical hatchling, 1/8 the size of the largest specimen.
Lepidosaurs generally carry their eggs longer within the mother
than birds or crocs do, sometimes until the moment of hatching. This is not only a more parsimonious hypothesis based on actual evidence (“lizard-like eggshell thickness and leathery lizardy texture in pterosaur eggs”), but hypothetically hatching in open air, without the need to resurrect itself from burial is much to be preferred in tiny pterosaurs with fragile, bat-like wing membranes… much more fragile than wet hatchling bird feathers found in precocial mound building birds. And what happen to pliable eggs that are buried. Might get a little tight inside those eggshells! Mom, on the other hand, always makes room for her growing babies in their eggs.
Unwin and Deeming supplementary material identifies
without phylogenetic analysis, a list of small pterosaurs the authors label juveniles. After phylogenetic analysis, many of these turn out to be small adults (Peters 2007 and here). Earlier we talked about pterosaur workers putting on their blinders and ruining/distorting our understanding of pterosaurs by employing taxon exclusion in phylogenetic analysis. Alas, it has happened once again.
If you are wondering,
I submitted a paper (now available on ResearchGate.net here) on isometric pterosaur growth patterns. The pterosaur referees rejected it for reasons that are clear given the present attitude toward conflicting hypotheses.
References
Bennett SC 1996. The phylogenetic position of the Pterosauria within the Archosauromorpha. Zoo. J. Linn. Soc. 118, 261–308.
Bennett SC 2012/13. The phylogenetic position of the Pterosauria reexamined.
Hist. Biol. 25, 545–563.
Peters D 2000. A Redescription of Four Prolacertiform Genera and Implications for Pterosaur Phylogenesis. Rivista Italiana di Paleontologia e Stratigrafia 106 (3): 293–336.
Peters D 2007. The origin and radiation of the Pterosauria. Flugsaurier. The Wellnhofer Pterosaur Meeting, Munich 27
Unwin DM and Deeming DC 2019. Prenatal development in pterosaurs and its implications for their postnatal locomotory ability. Proceedings of the Royal Society B https://doi.org/10.1098/rspb.2019.0409
Note: Prior to understanding pterosaurs were lepidosaurs, I fell back upon tradition and labeled the outgroup taxa ‘prolacertiforms’ in Peters 2000, to my eternal embarrassment and with the approval of my editors and referees. Worse yet, today, twenty years later, most workers are still caught up in that error. Phylogenetic analysis solves so many problems. It’s really a cure for nearly everything in paleontology.
From the NYTimes.com article:
“Other experts were convinced by the paper’s assessment of embryo development, but not its behavioral conclusions.
“In order to prove those, the study would need to compare the pterosaurs with megapodes, chicken-like birds from Australia that can fly from birth, said Edina Prondvai, a postdoctoral researcher at Ghent University in Belgium and the MTA-MTM-ELTE Research Group for Paleontology in Budapest. Kevin Padian, a biologist at the University of California, Berkeley, called the idea that hatchlings could support their own body mass in the air “quite a stretch,” based on studies of birds.
“Dr. Unwin replied that he would have liked to compare pterosaurs with megapodes, but could not find enough data, and that “pterosaurs are not birds.
“He prefers it that way.
“It’s that sheer alienness of pterosaurs that is really fascinating about them,” Dr. Unwin said. “These were creatures that were really different than anything that’s around today.”
No, they are like lizards, bipedal lizards…(Peters 2000)
because they are lepidosaurs (Peters 2007). That has been validated by taxon inclusion. something Unwin and Deeming are loathe to do. Studying megapodes would be a waste of time, based on phylogenetic bracketing.