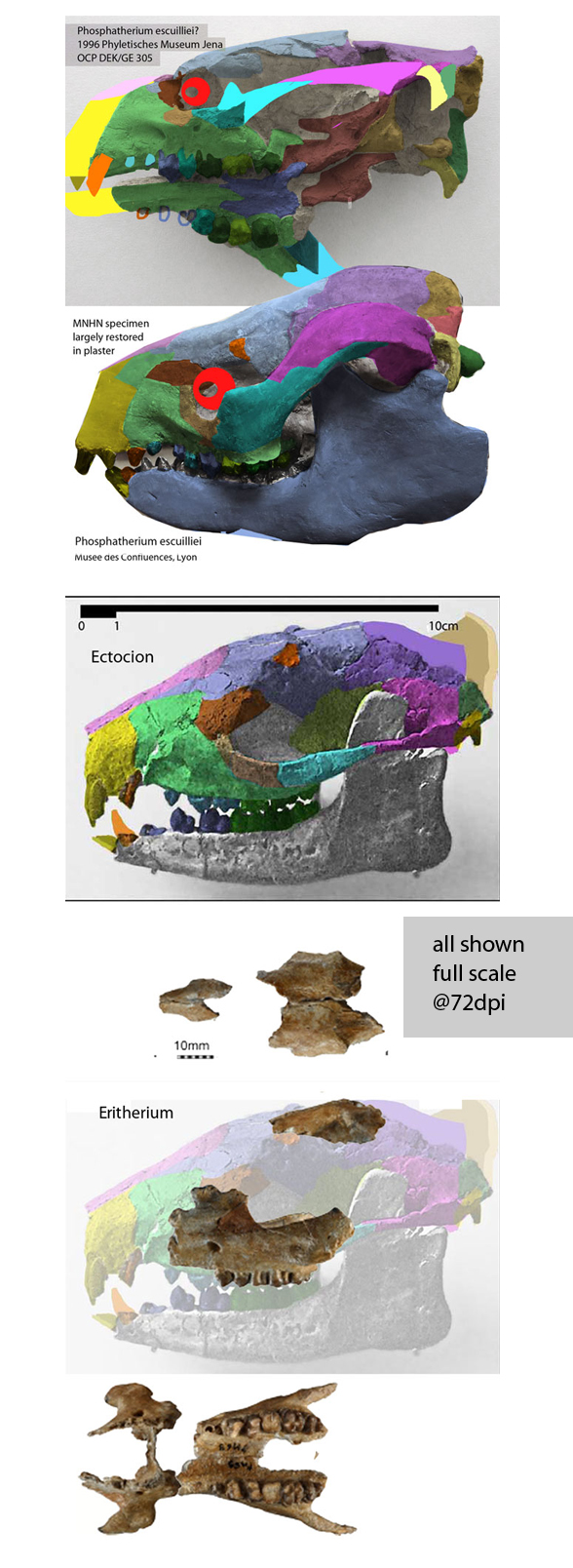
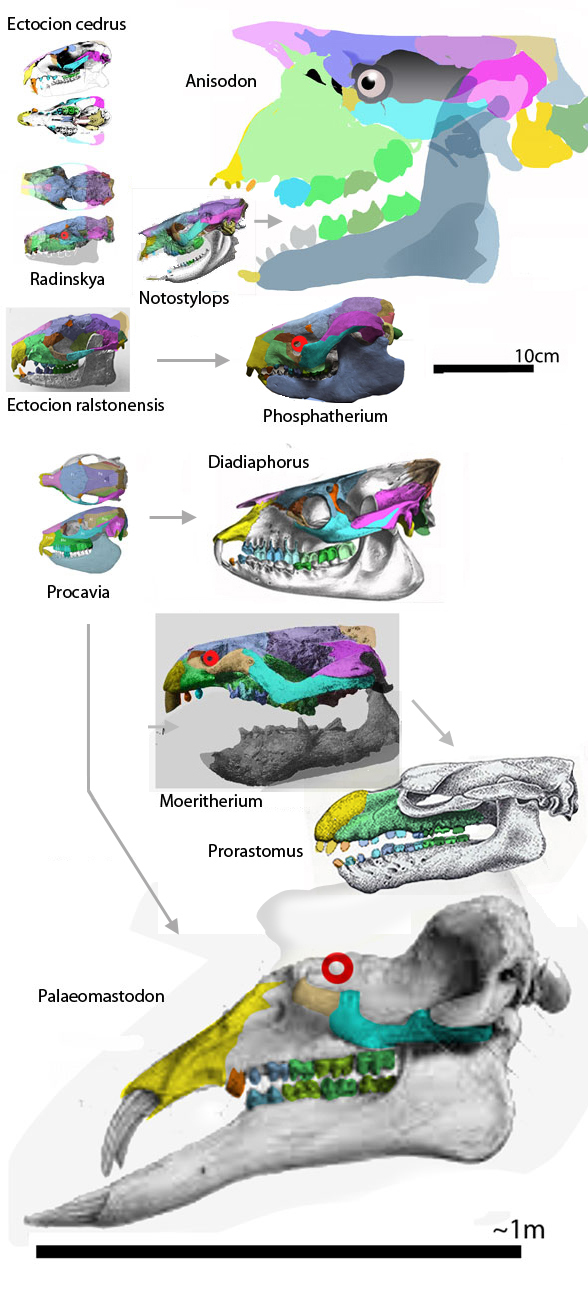
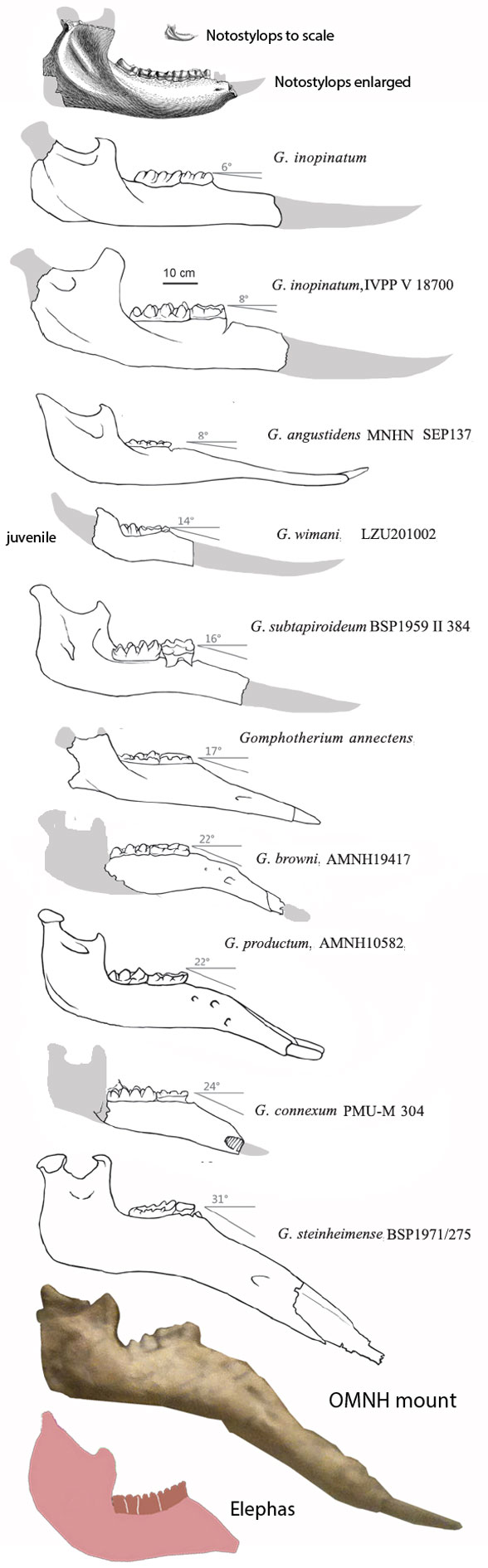
Few placental clades are as misunderstood as the Desmostylia.
This was well documented by Domning, Ray and McKenna 1986 with their review of desmostylian origin (Figs 1, 3) and interrelation studies (abbreviated below). This timeline provides insight into the phylogenetic struggles paleontologists suffered over this clade in the pre-cladistic, pre-software era.

Unfortunately, this struggle continues in 2022
due to… (drum roll, please) …taxon exclusion. You’ll either weep or chuckle with bemusement as the best PhDs of their respective eras kept their blinders on, spending decades struggling for an answer that wasn’t going to be found where they were looking. Apparently most workers were hesitant to look somewhere other than where others had looked for a solution to the 134-year-old desmostylian problem. Note how professors through the decades kept beating around the same bush without success or resolution, not imagining they should be looking elsewhere.
Abridged from Domning, Ray and McKenna 1986:
“O.C. Marsh (1888) referred Desmostylus to the Sirenia.”
“Yoshiwara and Iwasaki (1902), believed their find [a desmostylian] to be some sort of proboscidean, based in part on a letter from H.F. Osborn. Although Osborn had “informed” them that the skull belonged to a proboscidean, Yoshiwara and Iwasaki demonstrated that it was not like deinotheres or elephantids and therefore would have to represent a branch from the primitive proboscideans, near the origin of that order from among the other ungulates. They also mentioned some similarities to Sirenia.”
“Hay (1915), followed by Matsumoto (1918), placed the Desmostylidae in the Sirenia, although
he emphasized that the Desmostylidae were very different from other (true) sirenians.”
“Abel (1922:381; 1923) abandoned his view of proboscidean affinities of Desmostylus in
favor of a bizarre notion that it belonged to the mammalian subclass Allotheria (= Multituberculata).”
Although misguided and wrong, at least Abel was expanding his gamut.
“Sickenberg (1938), however, argued strongly against a desmostylian-sirenian relationship.”
Ijiri (1939) considered Desmostylus to be an ungulate “in the broadest sense” but not a monotreme, multituberculate, marsupial, or sirenian.”
Minkoff (1976) suggested that desmostylians should be placed with the Amblypoda rather
than the Paenungulata.
Amblypoda are basalmost condylarths = Pantodontids and Phenacodontids in the LRT. Like others before, Minkoff was likewise playing darts in the dark by suggesting and guessing.
Domning, Ray and McKenna 1986 correctly compared desmostylians with hippos,
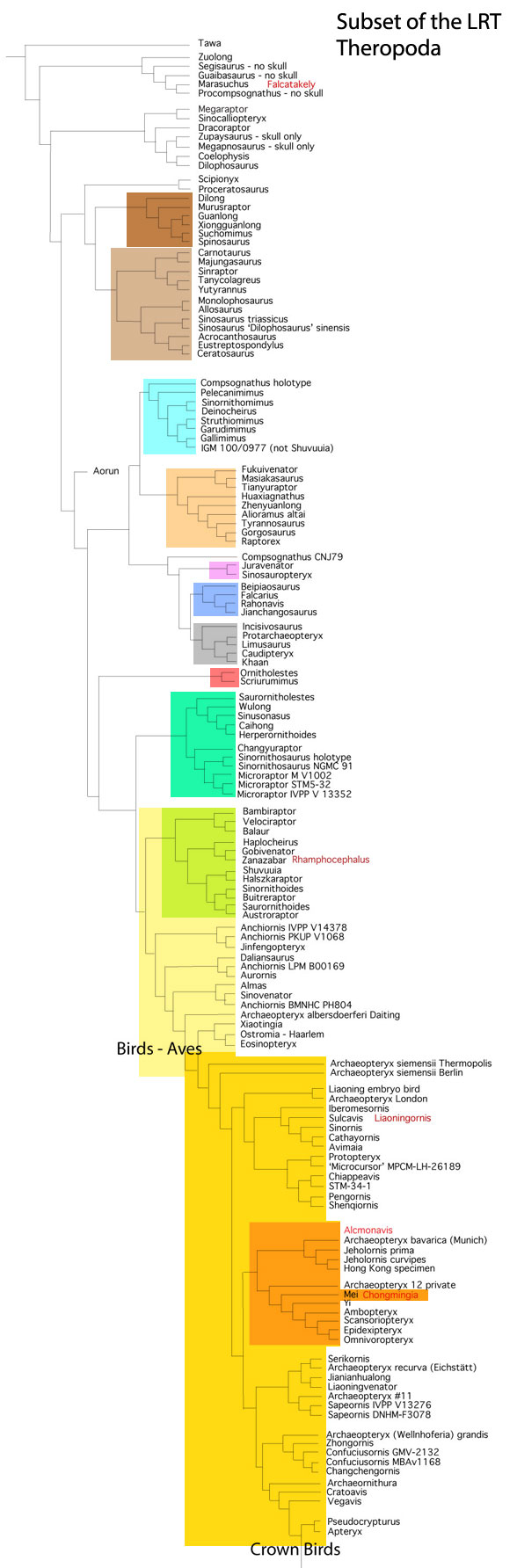
but ironically did not permit hippos to enter analysis. The authors made no mention of the mysticeti, mesonychids, or oreodonts, which turn out to be related to desmostylians when more taxa are added to analysis, as in the large reptile tree (LRT, 2061 taxa, subset Fig 2).

But wait, there’s more…
Workers often and correctly associate desmostylians with anthracobunids, but once again, fall into the same proboscidean sticky trap.
Domning, Ray and McKenna 1986 considered Anthracobune a proboscidean,
so their phylogenetic problems extended beyond desmostylians. In the LRT (Fig 2) anthracobunids nest between hippos and desmostylians, far from elephants.
From Domning, Ray and McKenna 1986:
“Manning’s identification of Anthracobune as a Moeritherium-Vike animal was also generously made known to R.M. West, who was the first to publish on the matter (West, 1980:518; 1983). West placed Anthracobune in the Moeritheriidae.”
“During the long interval from 1940 to 1980, Anthracobune (with “Pilgrimella”) had masqueraded as an artiodactyl (Pilgrim, 1940; Gingerich, 1977; Coombs and Coombs, 1977; and most other authors), a perissodactyl (Coombs and Coombs, 1977:303; 1979), and a phenacodontid condylarth (Van Valen, 1978, fig. 3). Wells and Gingerich (1983) assigned it to a new family Anthracobunidae within the Proboscidea, and (based on an examination of the specimens of Behemotops reported herein) suggested that the Desmostylia, as well as the Moeritheriidae and the Sirenia, may be derived from anthracobunids. The fact that Anthracobune occurs in southern Asia, rather than in Africa, was doubtless a major cause of its long neglect in discussions of the phylogenetic origin of proboscideans and their possible affinities to the desmostylians.”
Lessson 1: Let your software and a wide gamut taxon list
tell you which taxa to include, not the other way around. That would be considered akin to gerrymandering or cherry-picking. And it’s okay to make mistakes along the way. Look at the professionals of the past that kept their blinders on despite their lack of confidence in their results (see above). None were ostracized or lost their paycheck or place in science.
Lesson 2: Don’t depend on dental traits at first,
not until your wide gamut cladogram is fully resolved and major clade interrelationships are confidently known from substantial skulls and skeletons. Then you can drop in your partial taxa and dental taxa with greater confidence.

If you are at all prescient, or a long time reader,
you’ll know what comes next: Pterosaurs. Turtles. Snakes. Reptiles. Placoderms. Creodonts. Bats. Catfish. Diapsids. These are all taxa that find a good, secure (= fully resolved) home in the LRT, something they cannot presently find in university textbooks. Desmoystlians are also firmly nested in the LRT, but still an enigma otherwise.
Remember, all professors were once students
eager to please their own professors by parroting paradigms. All academic authors know they have to satisfy anonymous referees (= sometimes jealous and self-serving academic competitors). Both systems discourage discovery and heresy. In academia consensus rules (see timeline above). It always has. And it always will. That means it comes down to politics, not science. That’s why it sometimes takes an outsider to pull the curtain back on enigma taxa to give them a home based on the scientific method with complete transparency and falsifiability …and to take the occasional barb for the trouble of doing so without payment or tangible reward.
References
Domning DP, Ray CE and McKenna MC 1986. Two New Oligocene Desmostylians and a Discussion of Tethytherian Systematics. Smithsonian Contributions to Paleobiology 59:56pp.
Peters D unpublished. The triple origin of whales. PDF on ResearchGate.org