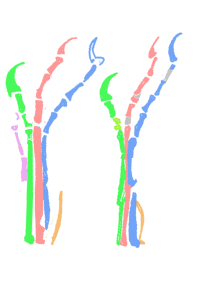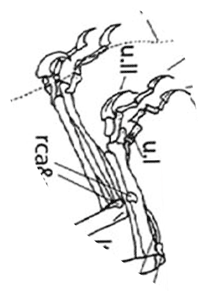
Updated April 18. 2020
The four-fingered manus tracks (identified below out of context as a rhamphorhynchid pes track) belong to a tenrec, not a pterosaur. Details here.
Mazin and Pouech 2020
report on basal pterosaur tracks from the “Pterosaur Beach of Crayssac” (Upper Jurassic), which they consider novel.
From the abstract:
“New discoveries on the ichnological site known as “the Pterosaur Beach of Crayssac” (lower Tithonian, Upper Jurassic; south-western France) answer the question of terrestrial capabilities of non-pterodactyloid pterosaurs. If the terrestrial type of locomotion of pterodactyloid pterosaurs has been solved from ichnological evidence for more than twenty years, no tracks and trackways referable to non-pterodactyloid pterosaurs have ever been described.”
Not true. Peters 2011 included several anurognathid tracks and matched them to trackmakers (Fig. 1). We looked at the so-called ‘Sauria aberrante‘ from Patagonia earlier here in 2011.
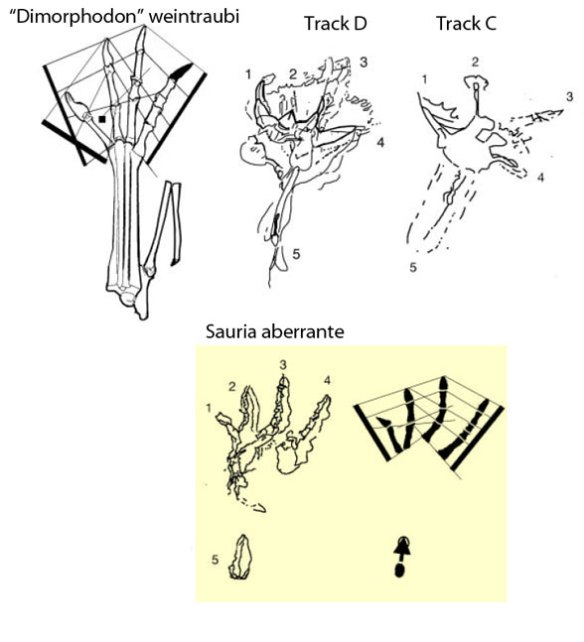
Figure 1. A pterosaur pes belonging to a large anurognathid, “Dimorphodon weintraubi,” alongside three digitigrade anurognathid tracks and a graphic representation of the phalanges within the Sauria aberrante track.in
Continuing from the abstract:
“Thus, the debate on terrestrial capabilities of these non-pterodactyloids was based on morpho-functional studies, with the main conclusion that those pterosaurs were arboreal dwellers and bad walkers.”
Not true. Peters 2000a, b, 2011, demonstrated a bipedal ability in pterosaurs superior to that of extant bipedal lizards, (e.g. Chlamydosaurus).
The ‘bad-walker myth’ results from mythology promoted by Unwin and Bakhurina1994 with regard to several misinterpretations of Sordes pilosus. including the invalid binding of the hind limbs with a uropatagium along with the invalid continuation of the brachiopatagium trailing edge to the ankle.

Figure 2. Dimorphodon pes with shadows. Pedal digit 5 can swing beneath the metatarsus. Note elevated proximal phalanges.
“Six trackways referable to three non-pterodactyloid new ichnotaxa, maybe closely related to Rhamphorhynchidae, are described in this work. Their study leads to the conclusion that grounded non-pterodatyloids, at least during the Late Jurassic, were quadrupedal with digitigrade manus and plantigrade to digitigrade pes.”
This confirms work by Peters 2000a, b, 2011.
“They were clearly good walkers, even if hindlimbs are supposed to be hampered by the uropatagium, what could have constrained the terrestrial agility of these animals.”
A single binding uropatagium is a myth invalidated several years ago. See above.
“Thus, from ichnological evidence and contrary to the current hypotheses, non-pterodactyloid pterosaurs seem to have been good walkers even though their trackways are very rare or unidentified to date.”
This also confirms work by Peters 2000a, b, 2011.

Figuue 3. Cosesaurus matched to Rotodactylus from Peters 2000.
Continuing from the abstract:
“This rarity could be due to behaviour rather than to functional capacities, many non-pterodactyloids being considered both littoral fishers and arboreal or cliff dwellers. However, the concept of non-pterodactyloid “good climbers and bad walkers” has to be modified to “good climbers and rare walkers”, unless many non-pterodactyloid ichnites have yet to be discovered.”
Many non-pterodactyloid ichnites have been discovered (Fig. 1). Unfortunately, they have been ignored and omitted by authors, including Mazin and Pouech. It’s never a good time to remember Dr. S. Christopher Bennett’s infamous threat, “You will not be published. And if you are published, you will not be cited.”

Figure 4. Crayssac track different from all others. Inset: Pes of Rhamphorhynchus muensteri JME-SOS 4009, no. 62 in the Wellnhofer catalog. NOTE ADDED APRIL 18, 2020. The Martin-Silverstone paper (link above) identifies this as a manus track. It belongs to a tenrec, not a pterosaur.
This used to be considered
crankery. Now they confirm the heretical hypotheses, but claim them as their own.

Figur 5. Unique among Rhamphorhynchus specimens, Rhamphorhynchus muensteri (Wellnhofer 1975) JME-SOS 4009, no. 62 in the Wellnhofer catalog has a long digit 4.
BTW
Earlier a published Craysaac a basal pterosaur track was matched to the pes of a particular Rhamphorhynchus (no. 62, JME-SOS-4009; Figs. 4, 5) in a 2011 blogpost on digitigrade pterosaur footprints. I heard of the Crayssac rhamph-tracks years ago and am glad to see their present publication. Still awaiting the paper. When it comes: more details.
NOTE ADDED APRIL 18, 2020. The Martin-Silverstone paper (link above) identifies this as a manus track. It belongs to a tenrec, not a pterosaur.

Figure 6. Cosesaurus and Rotodactylus, a perfect match. Elevate the proximal phalanges along with the metatarsus, bend back digit 5 and Cosesaurus (left) fits perfectly into Rotodactylus (right).
We also have tracks made by pre-pterosaur fenestrasaurs.
Rotodactylus, UCB 38023, Moenkopi Formation (Peabody,1948; Peters, 2000a; Figs. 3, 6)
References
Casamiquela RM 1962. Sobre la pisada de un presunto sauria aberrante en el Liassico del Neuquen (Patagonia). Ameghiniana, 2(10): 183–186.
Mazin J-M and Pouech J 2020. The first non-pterodactyloid pterosaurian trackways and the terrestrial ability of non-pterodactyloid pterosaurs. Geobios 16 January 2020. PDF
Peabody FE 1948.Reptile and amphibian trackways from the Lower Triassic Moenkopi formation of Arizona and Utah. University of California Publications, Bulletin of the Department of Geological Sciences 27: 295-468.
Peters D 2000a. Description and Interpretation of Interphalangeal Lines in Tetrapods. Ichnos 7:11-41.
Peters D 2000b. A Redescription of Four Prolacertiform Genera and Implications for Pterosaur Phylogenesis. Rivista Italiana di Paleontologia e Stratigrafia 106 (3): 293–336.
Peters, D. 2011. A Catalog of Pterosaur Pedes for Trackmaker Identification
Ichnos 18(2):114-141. http://dx.doi.org/10.1080/10420940.2011.573605
Unwin DM and Bakhurina NN 1994. Sordes pilosus and the nature of the pterosaur flight apparatus. Nature 371: 62-64.
Sauria aberrante MLP 61-IX-4-1 (Casamiquela, 1962)
Track D, Sundance Formation (Harris and Lacovara, 2004)
Track C, Sundance Formation (Harris and Lacovara, 2004)
https://pterosaurheresies.wordpress.com/2012/03/02/the-case-against-bipedal-pterosaurs
https://pterosaurheresies.wordpress.com/2011/08/09/pterosaurs-bipedal-quadrupedal-or-both/