Urochordates and larvaceans are the relatives
we almost never talk about. Here those long, lost relationships are reestablished.
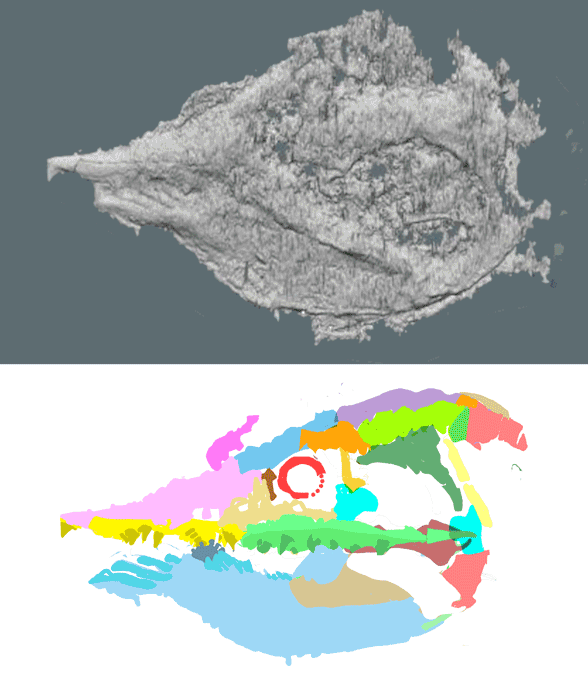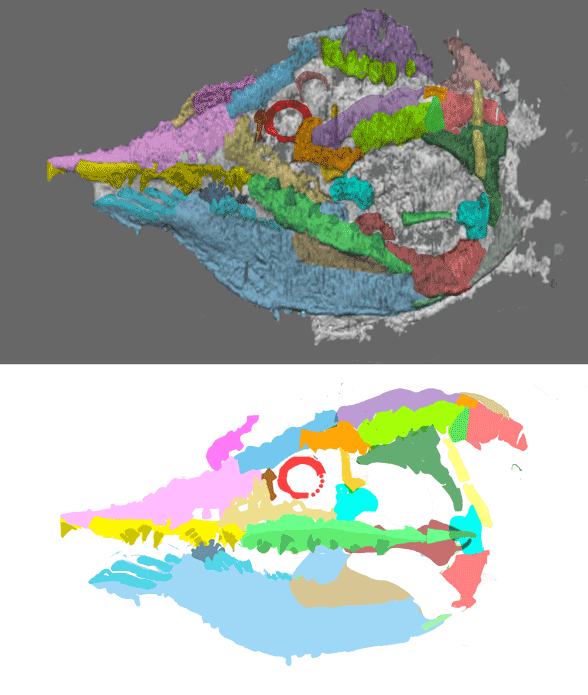
Didazoon haoae (Figs. 1, 2; Shu et al. 2001; ELI 0000197–0000217) was described as a member of the Ventulicolia, an “enigmatic phylum” of Cambrian deuterostomes of otherwise uncertain affinities. It was a simple, barrel-shaped animal with a line of gill openings dotting a large atrium followed by a down-angled tail. There was no head.
From the Diagnosis:
“Bipartite, cuticularized body, anterior segmented region and voluminous mouth, ventral margin ¯attened, widens posteriorly. On either side the anterior bears fve circular structures, in the form of a cowl with posteriorly directed opening and basin-like interior, apparently connected to interior. A prominent constriction separates dorsal region of anterior from posterior section. The latter, composed of seven segments, tapers in both directions, with rounded posterior termination. Internal anatomy includes alimentary canal, possibly voluminous in anterior, and in posterior narrow intestine, straight or occasionally coiled. Dark strand located along ventral side of anterior section, possibly representing endostyle.”

Figure 1. Didazoon in situ and reconstructed. The ‘intestine’ may be a notochord remnant because it extends to the tail tip, distinct from Metaspringgina (Fig. 2). The tail was down in all larvaceans.
Comparisons to Metaspriggina, Arandaspis and larvacea
(Fig. 2) were not made by Shu et al. 2001. Here the ‘cuticularized body’ is transitional between Branchiostoma (Fig. 2) and extant larvacea (Fig. 2 which share several traits with these vetulicolians.
In all these taxa, the tail hangs down
and the mouth opens slightly ventrally, beneath what was the rostrum in lancelets. Prior reconstructions at Wikipedia have the tail up and the animal upside-down.

Figure 2. Branchiostoma, Metaspriggina, Didazoon, Vetulicola, Arandaspis and Larvacea to scale. Note the presence of an atrial pore/posterior atrial opening in Branchiostoma and the vetulicolians while Metaspriggina and Arandaspis had fish-like gill openings. The tail of larvaceans hangs down.
According to Wikipedia,
Vetulicola (Figs. 2, 3) “is the eponymous member of the enigmatic phylum Vetulicolia, which is of uncertain affinities, but may belong to the deuterostomes. Vetulicola cuneata could be up to 9 cm long (shown at only 6cm in Fig. 2).
Earlier we looked at the parallel development of the enlarged gill chamber in craniates and gnathostomes like the manta ray (Manta) and whale shark (Rhinchodon) that also fed on plankton, but on a much larger scale.

Figure 3. Vetulicola in situ and flipped in vivo configuration. The shape is transitional to both the larvacea (Fig. 2) and to the crinoid (Fig. 5). Note the four-part separation of the mouth parts, like a budding flower.
According to Wikipedia,
“Vetulicola’s taxonomic position is controversial. Vetulicola cuneata was originally assigned to the crustaceans on the assumption that it was a bivalved arthropod like Canadaspis and Waptia, but the lack of legs, the presence of gill slits, and the four plates in the “carapace” were unlike any known arthropod.”
“Shu et al. placed Vetulicola in the new family Vetulicolidae, order Vetulicolida and phylum Vetulicolia, among the deuterostomes. Shu (2003) later argued that the vetulicolians were an early, specialized side-branch of deuterostomes.”
“Dominguez and Jefferies classify Vetulicola as an urochordate, and probably a stem-group appendicularian (= Larvacea, Tunicata). Like a common tunicate larva, the adult Appendicularia have a discrete trunk and tail.”
“In contrast, Butterfield places Vetulicola among the arthropods.”
“The discovery of the related Australian vetulicolian Nesonektris, from the Lower Cambrian Emu Bay Shale of Kangaroo Island, and the reidentification of the “coiled gut” of vetulicolians as being a notochord affirms the identification as an urochordate.”

Figure 4. Lavacea diagram showing the tadpole organism and the house it builds from cellulose and protein. This is a highly derived organism, with origins in the Cambrian with Vetulicola and close to the salp taxa in figure 3,. Here the larvacea retains the swimming muscles and notochord that extends to the tail tip.
According to Wikipedia:
“Larvaceans have greatly improved the efficiency of food intake by producing a test, which contains a complicated arrangement of filters that allow food in the surrounding water to be brought in and concentrated prior to feeding. By regularly beating the tail, the larvacean can generate water currents within its house that allow the concentration of food. The high efficiency of this method allows larvaceans to feed on much smaller nanoplankton than most other filter feeders.”
“The immature animals resemble the tadpole larvae of ascidians, albeit with the addition of developing viscera. Once the trunk is fully developed, the larva undergoes “tail shift”, in which the tail moves from a rearward position to a ventral orientation and twists 90° relative to the trunk. Following tail shift, the larvacean begins secretion of the first house.”
There are no other chordates that shift the tail 90º relative to the trunk and build an oceanic house of cellulose and protein. Nevertheless Vetulicolia is not a new phylum, but a transitional set of taxa linking lancelets to larvacea and to crinoids. Vetulicolians (Figs. 1–3) demonstrate the down-angled tail appeared in the Early Cambrian pointing to an Ediacaran origin for lancelet-like chordates. That means previously unknown, more active, less benthic (= sea floor) niche full of free-swimming chordates was also present in the Ediacaran.

Figure 5. Vetulicola compared to a fossil crinoid. Note the splitting of the mouth parts into separate ‘arms’ has only just begun here, starting with four. The crinoid stalk is the segmented ‘tail’ of Vetulicola.
When lips become arms… and tails become stems…
A clade of vetulicolians (former lancelets) also developed a down-hanging ‘tail’ (= stem) that ultimately evolved to become a holdfast: the crinoids within the echinoderms. Remember, adult lancelets also plant their tails in the substrate to become sessile plankton feeders. Crinoids are the ancestors of starfish (Fig. 6), a clade that flips, mouth-side down, and concurrently loses the tail (= stem). Not all echinoderms have a pentagonal morphology. The mouth of Vetucolia has four slightly separating mouth parts. The atrium and tail have just a trace of homologous armor, ringed in the case of the tail and stem.

Figure 6. Chordate evolution, changes to Romer 1971 from Peters 1991. Here echinoderms have a stem-like tail with a holdfast, later lost in starfish, similar to the tail and gills of the free-swimming tunicate larva and adult larvaceans.
Garcia-Bellido et al. 2014 concluded
“Phylogenetic analyses resolve a monophyletic Vetulicolia as sister-group to tunicates (Urochordata) within crown Chordata. The hypothesis suggests that a perpetual free-living life cycle was primitive for tunicates. Characters of the common ancestor of Vetulicolia + Tunicata include distinct anterior and posterior body regions – the former being non-fusiform and used for filter feeding and the latter originally segmented – plus a terminal mouth, absence of pharyngeal bars, the notochord restricted to the posterior body region, and the gut extending to the end of the tail.”
Garcia-Bellido et al. nested tunicates and vetulicolians as sisters to craniates, both derived from lancelets (cephalochordates). That seems to be correct based on the above figures in their tail-down orientation.
Cameron, Garey and Swalla (2000) reported,
“The nesting of the pterobranchs within the enteropneusts dramatically alters our view of the evolution of the chordate body plan and suggests that the ancestral deuterostome more closely resembled a mobile worm-like enteropneust than a sessile colonial pterobranch.”
Actually Peters 1991 (Fig. 6) published that hypothesis earlier… without the use of DNA.
Now we have long-sought evidence in the form of homologous traits that link vetulicolians to chordates (Fig. 2) and to echinoderms (Fig. 5). All have been understood as deuterostomes. The connection between larva was indicated earlier, according to Cameron, Garey and Swalla 2000. Now we have previously overlooked adult homologs to study and compare. This may be a novel hypothesis of interrelationships. If not, please provide a citation so I can promote it here.
Vetulicolians will not enter the LRT.
They diverge from the vertebrate lineage while keeping some basal lancelet traits and feeding patterns. The degree of their divergence indicates an ancient split from lancelets + fish, just as the divergence of starfish indicate a similar ancient split from lancelet deep time ancestors. They no longer resemble lancelets, except for their cirri-lined mouth parts and attendant mucous strands.
References
Aldridge RJ et al. (4 co-authors 2007. The systematics and phylogenetic relationships of vetulicolians. Palaeontology. 50 (1): 131–168.
Cameron CB, Garey JR and Swalla BJ 2000. Evolution of the chordate body plan: New insights from phylogenetic analyses of deuterostome phyla. PNAS 97(9):4469–4474.
Garcia-Bellido DC et al. 2014. A new vetulicolian form Australia and its bearing on the chordate affinities of an enigmatic Cambrian group. BMC Evolutionary Biology 2014, 14:214 http://www.biomedcentral.com/1471-2148/14/214
Peters D 1991. From the Beginning – The story of human evolution. Wm Morrow.
Shu D-G et al. (8 co-authors) 2001. Primitive deuterostomes from the Chengjiang Lagerstätte (Lower Cambrian, China) Nature 414:419–424.
wiki/Didazoon
wiki/Vetulicolia
wiki/Vetulicola
wiki/Larvacea
.https://www.youtube.com/watch?v=ZXCOZ2_blb8