This is when
paleontology goes awry.
Zhou, Miao and Andres 2023 examine the palate of a new ?Sinopterus
From the abstract:
“Here, we describe a new Jehol tapejarid skeleton tentatively referred to Sinopterus dongi, revealing some valuable cranial information about the palate and mandibular symphysis of this species.”
It’s a small roadkill fossil (Fig 1) twisted, crushed and missing large and small parts. Parts do not match those of Sinopterus dongi (Figs 2, 6 and see below).

The specimen foot
(Fig 2) is closer match to Tapejara than Sinopterus dongi. Both Tapejara and Sinopterus are derived from Huaxiapterus benxiensis (Fig 6) in the large pterosaur tree (LPT, 266 taxa). So we have a slight ID problem. All these taxa are pretty close to one another and nomenclature needs to better reflect phylogeny.

From the abstract:
“In the palate, the absence of the lateral process of the pterygoid indicates that the subtemporal fenestra is undivided, in contrast to the well-developed lateral process that subdivides the subtemporal and secondary subtemporal fenestrae in other azhdarchoids.
This paper focuses on the palate (Fig 3). Those “lateral processes of the pterygoid” in the example palates should have been labeled ectopterygoid, as in other pterosaurs. The terms ‘ectopterygoid’ and ‘palatine’ are not mentioned in the text – very strange for a focus on the pterosaur palate. This indicates a lack of understanding of pterosaur palate anatomy (Fig 4).
Tapejaridae are still not related to Azhdarchidae when more taxa are added to analysis. In the LPT the former clade arose from similar sharp-snouted, crested germanodactylids, shenzhoupterids and dsungaripterids (Fig 4). On the other hand, and far removed azhdarchids arose from traditionally omitted tiny dorygnathids. Adding omitted taxa reveals this topology. Following phylogenetic miniaturization, other traditionally omitted transitional pre-azhdarchids, gradually became taller, and more crane-like.

From the abstract:
“The mandibular symphysis is elongated to about 58.7% of the mandible length, significantly longer than the other two Jehol tapejarids (52.9–53.7%) and much longer than other tapejarids (38.0–51.0%), except for the basal tapejarid Caupedactylus ybaka (~65%).
In the LPT Caupedactylus is a derived tapejarid,
closer to Sinopterus lui and Tupuxuara (Fig 4).
“The symphyseal shelf is extensive, flattened, and sculptured by sinuous ridges and pits. The ratio of its length to the symphysis is 34.4%, between that of the possible tapejarid Bakonydraco galaczi (43.9%) and the coexistent chaoyangopterid Eoazhdarcho liaoxiensis (26.7%), and much distinct from the well-reduced condition in other tapejarids.
In the LPT Eoazhdarcho is transitional between germanodactylids
and shenzhoupterids + dsunageripterids + tapejarids.
“This discovery highlights the variation of the cranial morphology in the Tapejaridae and even the Azhdarchoidea.”
Workers are bound to find great variation whenever they try to combine unrelated taxa based on a single trait (the high dorsal margin of the antorbital fenestra) while omitting other taxa. Workers have cherry-picked taxa since 2003. Only the LPT employs 266 taxa while omitting many scrappy taxa known only from bits and pieces.

Whenever the pterosaur palate is mentioned,
Osi et al. 2010 is cited. They reported, “On the basis of a new, three-dimensionally preserved specimen of the Early Jurassic pterosaur Dorygnathus banthensis we present a reinterpretation of the pterosaur palate. The hard palate is formed by the extensive palatal plate of the maxilla and not by the palatine as has been generally reconstructed.”
This was old news in 2010, shedding no new light on the pterosaur palate.

As reported earlier a decade ago
ever since Williston (1902) and continuing through Huene (1914), Wellnhofer (1978, 1991) and Bennett (1991, 2001a,b), the solid palatal plate in pterosaurs has been considered the palatine (Fig. 1), but that was wrong. Zhou, Miao and Andres only mention Osi, Prondvai, Frey and Pohl 2010, ignoring earlier workers (listed below) who understood the pterosaur palate correctly.

Not the Palatine, the Maxilla!
Virtually ignored, Newton (1888), Seeley (1901 and Woodward (1902) reported that the solid palatal shelf in pterosaurs was an outgrowth of the maxilla, not the palatine. Unfortunately, I did not know that when I also reported (Peters 2000) that the palatal plate originated from the maxilla in Rhamphorhynchus.
I thought I had discovered something in Peters 2000, but that was freshman naiveté.
I was not yet aware of the earlier authors listed above. Evidently Osi et al 2010 were likewise unaware, since they claimed ‘a new interpretation’.
In some pterosaurs
(like Scaphognathus and Rhamphorhynchus) the much smaller palatine and ectopterygoid fused to form a single L-shaped element, the ectopalatine. This was due to their ancestry among fenestrasaurs and Macrocnemus (Fig 5), rather than archosaurs. In other pterosaurs the palatal elements migrated and elongated the ectopterygoid to originate on the parasagittal plane (Fig 4) apart from the palatine.
Zhou, Miao and Andres did not understand this
They failed to mention ‘the palatine’ and ‘the ectopterygoid,’ in their palate study. Instead they created a new misunderstanding of the pterosaur palate by fusing some elements and overlooking others. Pterosaur experts should become familiar with the Bauplan of the pterosaur palate (Figs 4, 5) rather than create new myths.
References
Bennett SC 1991. Morphology of the Late Cretaceous Pterosaur Pteranodonand Systematics of the Pterodactyloidea. [Volumes I and II]. – Ph.D. thesis, University of Kansas [Published by University Microfilms International/ProQuest].
Bennett SC 2001a, b. The osteology and functional morphology of the Late Cretaceous pterosaur Pteranodon. Part I and 2. General description of osteology. – Palaeontographica, Abteilung A, 260: 1-153.
Newton ET 1888. On the skull, brain and auditory organ of a new species of pterosaurian (Scaphognathus Purdoni) from the Upper Lias near Whitby, Yorkshire. Philosphoical Transaction of the Royal Society, London 179: 503-537.
Osi A, Prondvai E, Frey E and Pohl B 2010. New Interpretation of the Palate of Pterosaurs. The Anatomical Record 293: 243-258.
Peters D 2000. A Redescription of Four Prolacertiform Genera and Implications for Pterosaur Phylogenesis. Rivista Italiana di Paleontologia e Stratigrafia 106 (3): 293–336.
Seeley HG 1901. Dragons of the air. An account of extinct flying reptiles. – London, Methuen: 1-240.
Wellnhofer P 1978. Pterosauria. Handbuch der Paläoherpetologie, Teil 19.– Stuttgart, Gustav Fischer Verlag: 1-82.
Wellnhofer P 1991. The Illustrated Encyclopedia of Pterosaurs. London, Salamander Books, Limited: 1-192.
Williston SW 1902. On the skull of Nyctodactylus, an Upper Cretaceous pterodactyl. Journal of Geology 10:520–531.
Woodward AS 1902. On two skulls of Ornithosaurian Rhamphorhynchus. Annals of the Magazine Natural History 9:1.
Zhou C-F, Miao C and Andres B 2023. New data on the cranial morphology of the tapejarid Sinopterus from the Early Cretaceous Jehol Biota, Historical Biology, DOI: 10.1080/08912963.2023.2202219
Cau, Brougham and Naish 2015 say Balaur is a bird, contra the LRT
And yes, the usual adjective, ‘bizarre’ is in their headline.
Perhaps because Balaur has not one, but two ‘killer’ claws on the foot (Fig 1).

From the abstract
“The exceptionally well-preserved Romanian dinosaur Balaur bondoc is the most complete theropod known to date from the Upper Cretaceous of Europe.
Actually, only partly preserved and without a skull.
“Previous studies of this remarkable taxon have included its phylogenetic interpretation as an aberrant dromaeosaurid with velociraptorine affinities. Our reanalysis of two distinct phylogenetic datasets focusing on basal paravian taxa supports the reinterpretation of Balaur as an avialan more crownward than Archaeopteryx but outside of Pygostylia, and as a flightless taxon within a paraphyletic assemblage of long-tailed birds”.
The authors didn’t focus enough on basal paravian taxa. They employed only one of thirteen Solnhofen birds (Figs 3, 4). The LRT (Fig 2) uses nine.

From the abstract
“The placement among dromaeosaurids resulted in a suboptimal alternative that cannot be rejected based on the data to hand. Interpreted as a dromaeosaurid, Balaur has been assumed to be hypercarnivorous and predatory, exhibiting a peculiar morphology influenced by island endemism.
Assumed? Don’t assume hypercarnivory without a skull.
From the abstract
“However, a dromaeosaurid-like ecology is contradicted by several details of Balaur’s morphology, including the loss of a third functional manual digit, the non-ginglymoid distal end of metatarsal II, and a non-falciform ungual on the second pedal digit that lacks a prominent flexor tubercle.
None of this matters if the available traits nest Balaur with Velociraptor and untested Bambiraptor (Fig 2) in the LRT.
“Conversely, an omnivorous ecology is better supported by Balaur’s morphology and is consistent with its phylogenetic placement within Avialae.
By contrast, the large reptile tree (LRT, 2234 taxa, subset Fig 2) Balaur nests with dromaeosaurids, far from Avialae (starting with the Solnhofen birds).

From the abstract
“Our reinterpretation of Balaur implies that a superficially dromaeosaurid-like taxon represents the enlarged, terrestrialised descendant of smaller and probably volant ancestors.”
By contrast, the LRT (Fig 2) refutes these two competing against each other hypothesis of interrelationships (Figs 3, 4), perhaps only by adding pertinent omitted taxa. Scoring might also be an issue. For example, Cau, Brougham and Naish test only one Archaeopteryx (Figs 2, 3). But which one? The LRT tests nine Solnhofen birds. No two are identical. Their variety and importance was overlooked by the three authors and their two cladograms.
The dromaeosaurid, Bambiraptor, is missing from Cau, Brougham and Naish, but nests with Balaur in the LRT. That means it is important in this discussion.
LIkewise, Cau, Brougham and Naish test only one Compsognathus. The LRT tests both specimens, as well as a wide gamut of other pertinent taxa. More are better, as long as they are pertinent.

Cau, Brougham and Naish published competing cladograms
The LRT presents a third. Several LRT taxa omitted in the CBN cladograms should be included. As always, just a suggestion for improvement… perhaps next time.
Ideally scientists should be eager to add pertinent taxa to test and possibly improve their cladograms. Sadly, I don’t see that happen very often. Even when the two competing cladograms had different topologies, the three authors did not have the curiosity to find out and present why their results were not internally consistent. Be curious.
On a quasi-similar note: Discover Magazine online,
featured a story on independent preparator, Terry Manning, an important and controversial figure in paleo who also lacks a PhD. Professor David Unwin was quoted, “One of the issues is that Terry Manning has been seen as outside the discipline,” Unwin says today. “It’s a huge issue, an elitist attitude. We do tend to be an exclusive club, and that’s a problem.”
“Has been seen as outside” is the key statement. Unwin indicates it is up to academics to see contributors for their value, not to ignore, shun and disparage independent contributors. BTW, Unwin is no angel in this regard. As Unwin also indicates, no PhD embraces non-members of their “exclusive club,” no matter how many published papers or contributions. That seems to be because paleontology is a zero-sum game with a limited number of discoveries to be had out there – and PhDs want them all for themselves. They would and should have had the discoveries all to themselves, but they keep messing up, chiefly by taxon exclusion, as we see here in today’s blogpost.
References
Brusatte S, Lloyd G,Wang S and Norell M. 2014. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur–bird transition. Current Biology 24:2386–2392 DOI 10.1016/j.cub.2014.08.034.
Burnham DA, Derstler KL, Currie PJ, Bakker RT, Zhou Z and Ostrom J H 2000. Remarkable new birdlike dinosaur (Theropoda: Maniraptora) from the Upper Cretaceous of Montana. University of Kansas Paleontological Contributions 13: 1-14.
Cau A, Brougham T and Naish D 2015. The phylogenetic affinities of the bizarre Late Cretaceous Romanian theropod Balaur bondoc (Dinosauria, Maniraptora): dromaeosaurid or flightless bird? PeerJ 3:e1032; DOI 10.7717/peerj.1032
Csiki Z, Vremir M, Brusatte SL, Norell MA 2010. An aberrant island-dwelling theropod dinosaur from the Late Cretaceous of Romania. Proceedings of the National Academy of Sciences of the United States of America 107 (35): 15357–15361.
Lee MSY, Cau A, Naish D and Dyke GJ 2014. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds.
Ostrom JH 1970. Stratigraphy and paleontology of the Cloverly Formation (Lower Cretaceous) of the Bighorn Basin area, Wyoming and Montana. Bulletin of the Peabody Museum of Natural History 35: 1–234.
discovermagazine.com
wiki/Balaur
wiki/Velociraptorwiki/Bambiraptor
Lessiniabatis: an Eocene stingray without a sting and not much of a tail enters the LRT
Marramá et al 2019 described,
“A dasyatoid stingray unique in having a thoracolumbar synarcual extending backward beyond the pelvic girdle, a tail that is extremely short and not protruding from the posterior edge of the pectoral disc, and pectoral radials that are proximally fused with one another.”
No surprise,
when added to the large reptile tree (LRT, 2234 taxa) Lessiniabatis (Fig 1) nests with Raja, the thornback ray (Fig 2).
References
Marramá G et al (4 co-authors) 2019. A bizarre Eocene dasyatoid batomorph (Elasmobranchii, Myliobatiformes) from the Bolca Lagerstätte (Italy) reveals a new, extinct body plan for stingrays. Nature scientific reports 9 article number 14087. https://doi.org/10.1038/s41598-019-50544-y
We have a ‘bear’ problem
Ursavus tedfordi
(Fig 1, Hofmann 1887) just entered the large reptile tree (LRT, 2233 taxa, subset Fig 2), but not where Qiu, Deng and Wang 2014 and later, Jiangzuo and Flynn 2020 (Fig 3), nested this Miocene fossil bear.
Traditional taxon exclusion explains the differences.

Figure 3. Ursavus nesrts between Nandinia and Ursus.
So does convergence.
According to the LRT (Fig 2), several subclades within Carnivora produced bear-morph taxa. Therefore it is incumbent upon the paleo-mammal community to recognize this. This can only be done by adding taxa, even if they don’t look like bears.
This is why we have a ‘bear’ problem, just like we have a ‘whale’ problem.
We have to look beyond the superficial.
The taxon may look like a bear and everyone might call it a bear, but many of these so-called ‘bears’ are not closely related taxa (Fig 2), contra prior topologies.

Ironically, under the rules of monophyly
every taxon more derived than Ursus arctos in the LRT (Fig 2) is also a bear. That clade includes cats, dogs, pinnipeds, wolverines, sabertooths and their kin. That clade does not include raccoons, skunks, civets and kin because those taxa are on the other branch of Carnivora (Fig 2).
So the Jiangzuo and Flynn cladogram is more or less upside-down, putting the most derived taxon, Canis, the wolf, at the outgroup node… all due to following and teaching from outdated textbooks at the university level.
Adding taxa is the solution. Build your own LRT.
Colleagues:
Add taxa to find out where taxa like ‘bears’ and ‘whales’ converge with superficially similar ‘bears’ and ‘whales’. Do not assume monophyly based on superficial traits. Let your own LRT tell you how taxa are related, or not related to one another. Don’t cherry-pick your taxon list. Don’t leave this in the hands of amateurs.
As you can see, adding characters will not help.
Add taxa. That’s why the LRT was built, to minimize taxon exclusion and shed new light on hypothetical interrelationships. Now the LRT needs competing analyses to confirm, refute or modify its tree topology. Don’t wait for approval from your professor. Do this on the side if you have to.
References
Hofmann A 1892. Beiträe zur miocaenen Säugetierfauna der Steiermark. Jahrb Geol Reichanst, 42(1): 63−76
Jiangzuo Q and Flynn JJ 2020. The earliest ursine bear demonstrates the origin of plant-dominated omnivory in Carnivora. IScience CellPress doi.org/10.1016/j.isci.2020.101235
Qiu Z-X, Deng T and Wang B-Y 2014. A late Miocene Ursavus skull from Guanghe, Gansu, China. Vertebrata PalAsiatica 52(7):265–302.
Hone 2023 looks at the pterosaur ‘sternum’ (= sternal complex)
Somehow
Hone 2023 managed to shed even more darkness on the subject of pterosaurs. This time he considered the sternal complex (= sternum + interclavicle + clavicles, Wild 1993, Fig 1), which Hone called the sternum. Hone combed the literature, examined many specimens and reported what he found, unfortunately without offering any new valid insights. Several times he indicated he was at a loss to explain several basic facts about pterosaurs (see below).
This is never good for someone who considers himself a pterosaur expert.
Example:
“There is only one well-preserved sternum known for any anurognathid and that is in Batrachognathus.”
This is incorrect. Most preserve the sternal complex (Fig 4). Those few that don’t are often exposed in dorsal view with the sternum still buried in the matrix below the ribs awaiting a µCT scan. Unfortunately, Hone is well known for his less than precise tracings and observations (see list below) and unfortunately this pattern continues in this present paper.
Hone 2023 reports,
“The ancestry of pterosaurs has been controversial and difficult to resolve for an extended period (Hone and Benton, 2007; Bennett, 2013b),
Hone is at fault for this as he considered, then ignored the older literature on pterosaur origins (Peters 2000a, b, 2002) ironically featured front and center in Hone and Benton 2007, 2008. So this is a sin of commission, not an accidental oversight. In his 2023 paper Hone also ignored more recent literature (e.g. Peters 2007, 2009, 2011) on pterosaur origins and has rejected a more recent manuscript (2018) available at ResearchGate.net on the subject.
Hone 2023 continued,
“…but it has recently become clear that pterosaurs are derived archosaurs and lie close to the origin of the Dinosauromorpha.”
Ironically… Hone allied pterosaurs with lepidosaurs on his figure 18 (Fig 2 below) when he presented a Varanus pectoral girdle with an interclavicle and a dinosaur pectoral girdle without one. Hone reported, “I suggest that the interclavicle was smaller and instead forms the cristospine [of the pterosaur sternal complex] alone,” apparently not realizing his inconsistent hypothesis of interrelationships AND taking credit for something that has been known since Wild 1993.

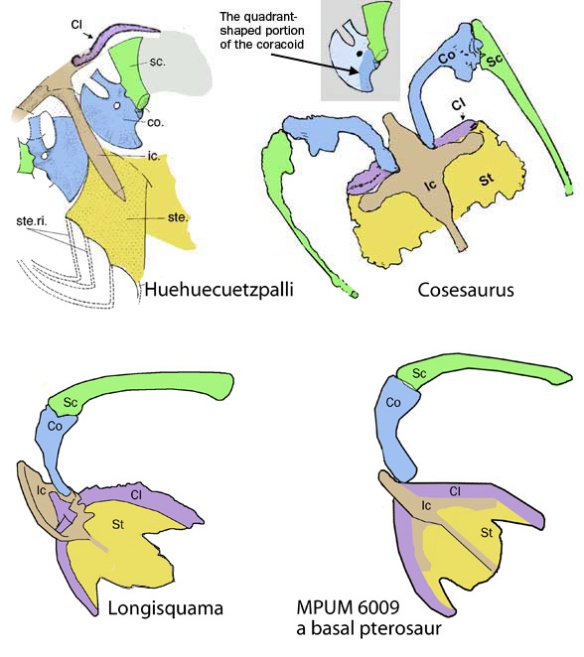
Not sure why Hone traditionally ignores valid and tested pterosaur outgroups,
like Longisquama and Cosesaurus (Figs 1, 3), that show the pre-sternal complex developing in lepidosaur pterosaur ancestors. Evidently Hone prefers what he was taught in grad school, perhaps not realizing that discovering things like pterosaur ancestors is >his job<. How can Hone claim to be a specialist if Hone has no idea what pterosaurs are or how they are related to other taxa? Sadly, that’s just the start of Hone’s long history of mishaps (see below).

Hone reported,
“The intrarelationships of the pterosaurs remain somewhat uncertain, and controversial and multiple competing hypotheses exist for their phylogeny. It is even unclear for some taxa to which major clade they belong, and other clades may join or leave different branches of the pterosaur tree in different analyses. As such, some of the assignments of taxa used throughout this paper remain uncertain or controversial, and thus it is also difficult to discuss.”
A wide gamut phylogenetic analysis built by Dr Hone would have resolved these many phylogenetic problems. Alas, we’re all still waiting for this.
At the same time, Hone omitted citing any peer-review papers by Peters, who published on pterosaur origins and interrelations several times over the past two decades.
Putting that aside, this is Hone’s job, as a paid pterosaur specialist, to understand the origin and interrelationships of pterosaurs. If interrelationships are indeed uncertain, Dr David Hone should fix that. Otherwise unpaid amateurs will fill this void.
Advice for Dr. Hone:
Stop whatever you’re doing and start building your own LRT. This will answer your questions and provide solutions to your prehistoric problems… or at least give you a good start. Trait-based phylogenetic analysis has revolutionized paleontology and is the foundation for all subsequent hypotheses.
Stop omitting literature that doesn’t fit whatever you were taught under the tutelage of your professor, Mike Benton. You are no longer under his spell. If you disagree with published literature on the origin of pterosaurs, publish a valid argument with a similar taxon list.
Don’t be afraid to trace fossils with colors and reconstruct them. The details you too often overlook (Fig 4) too often matter. This is where the fun is. Rebuilding taxa once lost in time.
David, you spent good money and precious time on your education under the influence of professor Benton. We’re still waiting for you to do something wonderful and valid. You’re young, brash and intelligent. You should be leading the charge, building a list of discoveries, not mired in a list of mistakes. Somehow you have managed to shed darkness on pterosaur anatomy by reporting pterosaurs had a mandibular fenestra, an antorbital fossa, wing membranes that extended to the ankles, wing tips that curved anteriorly. You embraced the quad launch hypothesis, the pterosaurs are archosaurs hypothesis, the allometry of juveniles and agreed with the owl-eyed anurognathid hypothesis. We have yet to see you conduct a wide-gamut phylogenetic analysis or create a precise reconstruction based on your own observations. Unfortunately, you and your old professor Benton will always be known as the two PhDs who un-discovered the origin of pterosaurs. Bennett 2013 called out your mistakes, which you never corrected or acknowledged. Every time you publish you’re twenty years behind the times or you are again creating confusion (Fig 2).
Stop bowing to peer group pressure. You are a scientist! Think for yourself even if that makes you a renegade or a heretic. You’re a PhD and have been for over a decade. You’re well connected. Every museum either knows you or expects to see you soon. It’s time to break out and do something great, or at least valid and novel. Your many past mistakes will then be forgiven, but never forgotten. Unfortunately, that’s your albatross to wear eternally. You took the wrong advice too often when you were young and this pattern of always making the wrong choice has to stop if you are going to make any real progress.
Even so, there’s hope for you yet. Your career should last for several more decades.
Readers: Consider Dr. Hone’s many reports with the tacit understanding
that he traditionally omits pertinent taxa and key literature, generally avoiding his duty to confirm, refute or modify published hypotheses whenever necessary. If Hone disagrees with a hypothesis he generally ignores it rather than refuting it, even though >that’s his job<. In other words, like so many other pterosaur workers, he keeps his blinders on.

By contrast,
the LRT and LPT provide authority and evidence to confirm, refute and modify hypothetical interrelationships. If those are wrong or need modification, I hope other workers will soon show exactly where the problems are with a similar taxon list. Corrections here are a constant. Likewise, DGS tracings provide ideal evidence for identifying elements in the matrix. We should all be coloring every bone in the matrix with a standard palate. Then these can be repaired and corrected whenever necessary… with cooperation, not animosity.

PS
Figure 19 of Hone 2023 presents the ‘sternum of a juvenile Altmulopterus‘. Google does not list this taxon. It might be a typo. If so, it could be Altmuehlopterus rhamphastinus formerly Germanodactylus rhamphastinus (Wagner 1851 B St AS I 745, No. 64 of Wellnhofer 1970), which is not a juvenile.
References
Bennett SC 2013. The phylogenetic position of the Pterosauria within the Archosauromorpha re-examined. Historical Biology 25(5-6): 545-563.
Hone DWE 2023. The anatomy and diversity of the pterosaurian sternum. Palaeontologia Electronica Article number: 26.1.a12 https://doi.org/10.26879/1261
Hone DWE and Benton MJ 2007. An evaluation of the phylogenetic relationships of the pterosaurs to the archosauromorph reptiles. Journal of Systematic Palaeontology 5:465–469.
Hone DWE and Benton MJ 2008. Contrasting supertree and total evidence methods: the origin of the pterosaurs. Zitteliana B28:35–60.
Peters D 2000a. Description and Interpretation of Interphalangeal Lines in Tetrapods. Ichnos 7:11-41.
Peters D 2000b. A reexamination of four prolacertiforms with implications for pterosaur phylogenesis. Rivista Italiana di Paleontologia e Stratigrafia 106: 293–336.
Peters D 2002. A New Model for the Evolution of the Pterosaur Wing – with a twist. Historical Biology 15: 277-301.
Peters D 2007. The origin and radiation of the Pterosauria. In D. Hone ed. Flugsaurier. The Wellnhofer pterosaur meeting, 2007, Munich, Germany. p. 27.
Peters D 2009. A reinterpretation of pteroid articulation in pterosaurs. Journal of Vertebrate Paleontology 29: 1327-1330.
Peters D 2011. A Catalog of Pterosaur Pedes for Trackmaker Identification
Ichnos 18(2):114-141. http://dx.doi.org/10.1080/10420940.2011.573605
Wild R 1993. A juvenile specimen of Eudimorphodon ranzii Zambelli (Reptilia, Pterosauria) from the upper Triassic (Norian) of Bergamo. Rivisita Museo Civico di Scienze Naturali “E. Caffi” Bergamo 16: 95-120.
Yesterday’s Gracilicollum enters the LRT with Macrocnemus, not with Tanystropheus, despite the convergent hyper-long neck
Wang et al 2023 tested and nested
long-necked Gracilicollum (Fig 1) with another long-necked taxon, Tanystropheus. Wang et al noted the proportions, shapes and numbers of the cervicals were distinct from those of Tanystropheus, yet Wang et al nested the two taxa together with Tanytrachelos.

We looked at the discovery
and publication of Gracilicollum yesterday without conducting a competing phylogenetic analysis, but noted that only one of several species within several genera were tested. Here is that competing analysis with the addition of Gracilicollum (Fig 2). Results were surprising.

Today
Gracilicollum enters the large reptile tree (LRT, 2230 taxa) nesting with several specimens of Macrocnemus, not nearby Tanystropheus, one of several distantly related taxa with a longer-than-typical neck. As the LRT shows (subset Fig 2) , several clades evolved members that evolved longer than normal necks. Macrocnemus is a traditional member of the Tanystropheidae. 12 additional steps separate Gracilicollum from Tanystropheus.
This appears to be a novel hypothesis of interrelationships
awaiting confirmation, refutation or modification with a similar taxon list. Taxon exclusion continues to be the number one problem hindering the advancement of paleontology.
References
Wang et al (6 co-authors) 2023. A new long-necked archosauromorph from the Guanling Formation (Anisian, Middle Triassic) of southwestern China and its implications for neck evolution in tanystropheids. Anatomical Record (Hoboken) 2023. doi: 10.1002/ar.25216. Online ahead of print.
Gracilicollum: a new Middle Triassic tanystropheid, not an archosauromorph
Wang et al 2023 describe,
“a new long-necked archosauromorph… and its implications for neck evolution in tanystropheids.”
In the large reptile tree (LRT, 2228 taxa) tanystropheids (Fig 1) are lepidosaurs derived from an ancestor of Huehuecuetzpalli (Fig 1, Peters 2007).

Wang et al report,
“the evolutionary history of such hyper-elongated necks in these two archosauromorph clades remains unknown, partially because known close relatives such as Macrocnemus and Pectodens possess only moderately elongated necks.”
In the LRT Macrocnemus and Pectodens are also lepidosaurs and they provide a pretty good evolutionary history (Fig 1) contra Wang et al. Note how these seven authors dismiss these relatives in order to make their new find appear to be more important.
Wang et al continue,
“Here, we describe a newly discovered early diverging archosauromorph, Gracilicollum latens. Despite possessing a high number of cervical vertebrae, Gracilicollum gen. nov. is recovered as a tanystropheid in an evolutionary grade between Macrocnemus and Tanystropheus rather than as a close relative of Dinocephalosaurus, a result that is primarily attributable to the presence of palatal teeth and the anatomy of the cervical vertebrae in Gracilicollum gen. nov. [IVPP V 15636].
And suddenly Gracillicollum becomes an excellent transitional taxon between Macrocnemus and Tanystopheus. But which Macrocnemus specimen? Three are show above (Fig 1), and a wide variety are known (Fig 2), but not tested by Wang et al. Taxon exclusion bruises yet another otherwise wonderful report.
This might remind you of workers trained at universities
who use only one Solnhofen bird, when each one of thirteen is distinct and different. Tanystropheus likewise has several morphs. No two are identical.

On the plus side,
Wang et al colored the bones in situ (Fig 4).

On the negative side,
Wang et al used enough colors to suggest Christmas lights on the cervicals )Fig 4) and no colors when it came time to ID and reconstruct the skull elements (Fig 5).

Colleagues:
start bringing your A-game to professional publications. Don’t make amateurs point out your shortcomings. Add taxa. Reconstruct crushed elements.
References
Peters D 2007. The origin and radiation of the Pterosauria. In D. Hone ed. Flugsaurier. The Wellnhofer pterosaur meeting, 2007, Munich, Germany. p. 27.
Wang et al (6 co-authors) 2023. A new long-necked archosauromorph from the Guanling Formation (Anisian, Middle Triassic) of southwestern China and its implications for neck evolution in tanystropheids. Anatomical Record (Hoboken) 2023. doi: 10.1002/ar.25216. Online ahead of print.
Malacanthus now nests with Meiacanthus in the mahi-mahi clade
Quick one today
as the wolffish (Anarhichas, Fig 3) clade that includes Meicanthus (Fig 1) and Snyderidia, now nest in the mahi-mahi (Coryphaena, Fig 2) clade that includes Scomberoides and Malacanthus (Fig 1) in the recently updated large reptile tree (LRT, 2228 taxa).

At the same time
Notothenia and Callionymus now nests apart from these taxa, now closer to Galaxias.
Analysis permits and encourages learning something new every day,
chipping away at the all the problems in previous trees. Apologies for earlier errors. Solutions only become possible once they become paired up on screen among the 443 taxa, then re-scored and tested in analysis. Every taxon scored affects every other taxon. In ray-fin fish this has been like herding cats, which is why this round of housekeeping, which started in December is still not quite there even after several single trees in the meantime as part of the process.


Meiacanthus grammistes
(originally Blennechis grammistes Cuvier and Valenciennes 1836, Fig 1) is the striped poison-fang blenny. It lives in the warm Western Pacific and grows to 11cm in length. The lower jaw is notable for its large venomous upside-down saber teeth. Here it nests close to Malacanthus.
Malacanthus brevirostris
(Guichenot 1848; 30cm) is the extant quakerfish, a type of tilefish. A sharp spine grows from the operculum of this reef dweller. A traditional perciform, here Malacanthus nests close to Meiacanthus, a blenny. The parietal is fused to the frontal in these related taxa. They are typically living in pairs in sand burrows they have excavated. They feed on small fishes and invertebrates.
This appears to be a novel hypothesis of interrelationships.
If not, please provide a citation so I can provide it here.
References
Cuvier G and Valenciennes A 1836. Histoire naturelle des poissons. Tome onzième. Livre treizième. De la famille des Mugiloïdes. Livre quatorzième. De la famille des Gobioïdes. v. 11: i-xx + 1-506 + 2 pp., Pls. 307-343. [Valenciennes authored volume. i-xv + 1-373 in Strasbourg edition.]
Guichenot A 1848. Peces de Chile (In Gay, Claudio. Historia física v política de Chile. Paris & Santiago, 1848). (in Spanish).
de Lacepede BGE 1801. Histoire naturelle des poissons. 3: i-lxvi + 1-558, Pls. 1-34.
Linnaeus C 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata.
wiki/Mahi-mahi – Coryphaena
wiki/Malacanthus_brevirostris
wiki/Scomberoides
wiki/Meiacanthus
Strunius, Onychodus and Selenodus move to the Palaeoniscidae, far from the Sarcopterygii (pre-tetrapod lobe-fins)
Housekeeping continues as warranted
on the fish clades in the large reptile tree (LRT, 2228 taxa). Corrections continue. New light is shed on overlooked interrelationships as comparative anatomy provides instruction.
Strunius has always been an odd fit in the Sarcopterygii,
the clade of lobe fin fish that gave rise to tetrapods, twice. Like sarcopterygians: between the frontals and parietals there is a straight suture that becomes a gap. However, Strunius (Fig 1) has ray fins, not lobe fins. A medial tooth whorl (Fig 2) is present between the two dentaries not present in lobe-fin taxa. Dual external nares are present, rather than choanae (= posterior nares migration to the palate). And that straight suture: it’s a convergent trait in the LRT.

The Paleoniscidae has been a traditional problem clade.
Apparently few workers have been interested in working out their phylogeny. Here it is a transitional grade from spiny sharks to most bony fish.
As we learned earlier, several extant bony fish are more closely related to more primitive sturgeons, sharks and spiny sharks and convergently developed bony fish traits, overlooked prior to the LRT.

An outgroup taxon, Pteronisculus
(Fig 2) also has a straight suture between the frontals and parietals. Another ougroup taxon, Australosomus (Fig 2) has a precursor Strunius-like tripartite tail, no longer a heterocercal tail, as in the more primitive Pteronisculus.

This clade shift appears to be a novel hypothesis of interrelationships.
If not, please provide a citation so I can promote it here.
References
Andrews M, Long J, Ahlberg P, Barwick R and Campbell K 2006. The structure of the sarcopterygian Onychodus jandemarrai n. sp. from Gogo, Western Australia: with a functional interpretation of the skeleton. Transactions of the Royal Society of Edinburgh. 96 (3): 197–307.
Mondéjar-Fernández J 2019. A new onychodont (Osteichthyes; Sarcopterygii) from the Middle Devonian of Morocco and its bearing on early osteichthyan evolution. Journal of Systematic Palaeontology 0: 1–34. http://dx.doi.org/10.1080/14772019.2019.1655495
Gross W 1933 1936 Die Fische des baltischen Devons, Palcteontographica A 79:1-74.
Jessen 1966. in Piveteau (Ed.). Traite de paleontologie. Tome 4. L’origine des Vertebres, leur expansion dans les eaux douces et le milieu marin. Vol. 3. Actinopterygiens, Crossopterygiens, Dipneustes. Masson & Cie, Paris
Newberry JS 1857. Fossil fishes from the Devonian rocks of Ohio. Geological
Survey of Ohio: Bulletin National Institute: 1–120.
Schultze H-P 1973. Crossopterygier mit heterozerker Schwanzflosse aus dem Oberdevon Kanadas, nebst einer Beschreibung von Onychodontida-Resten aus dem Mitteldevon Spaniens und aus dem Karbon der USA. Palaeontographica, Abteilung A, 143:188–208.
wiki/Strunius
wiki/Onychodus
wiki/Selenodus – not listed yet
wiki/Onychodontiformes
wiki/Sarcopterygii
wiki/Palaeonisciformes






