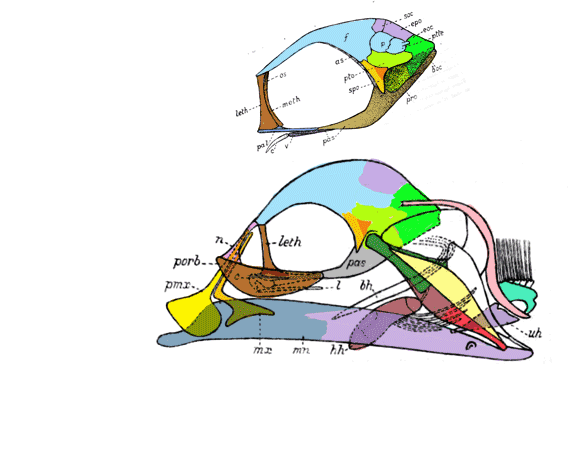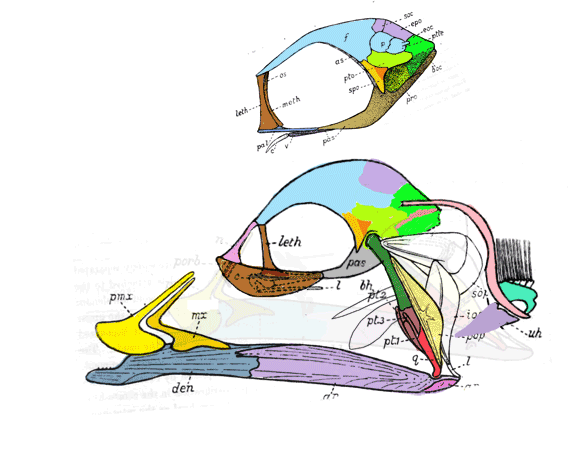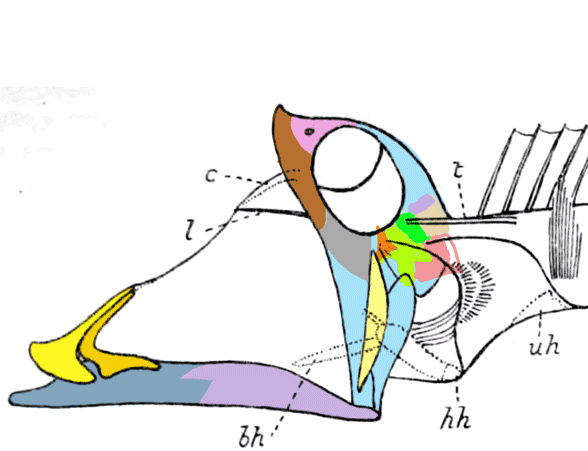
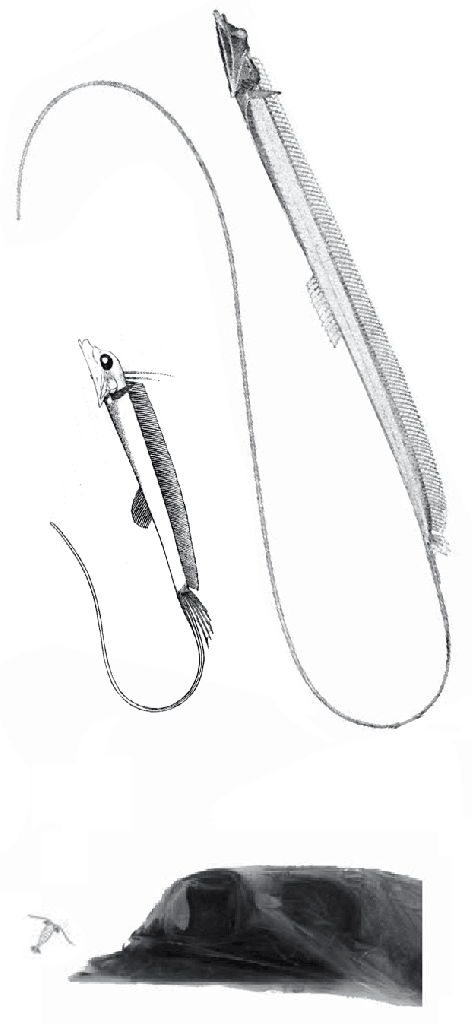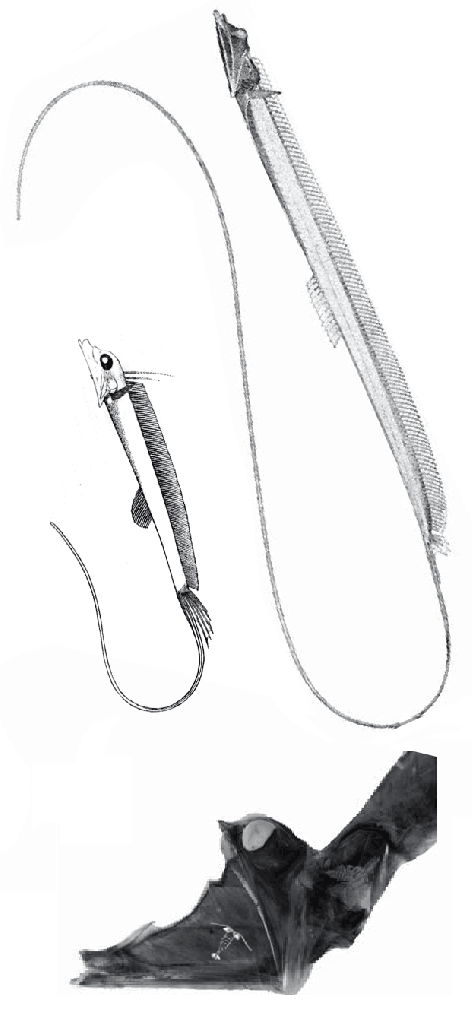
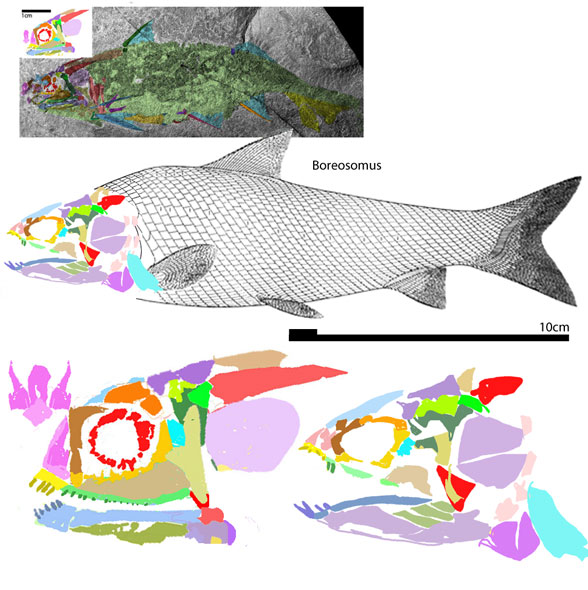
The fragile facial bones of ray-fin fish
are important when it comes to scoring them in the large reptile tree (LRT, 2223 taxa). So when they get jumbled, as in this cadaver (Figs 1, 2), it’s a good idea to use Photoshop to put the bones back to their original order and in vivo positions. Then score the traits.
To do this it with confidence takes a little experience to recognize previous errors and a good model, a Bauplan from a related taxon, to guide your changes.

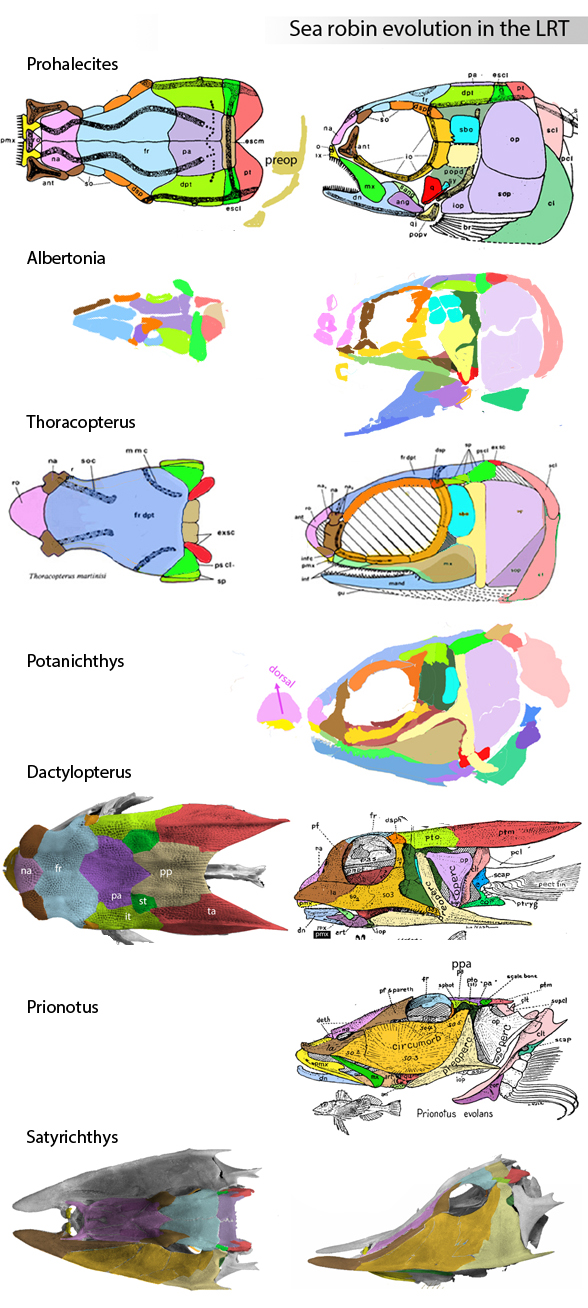
In this case the model for the 140cm long extant slickhead, Narcetes
(Fig 1) is a tiny 4cm long Middle Triassic basal bony ray-fin fish, Prohalecites (Fig 3). These two nest together in the LRT.

Narcetes shonanmaruae
(Alcock 1890; Fujiwara et al. 2021; 140cm, typically 75cm) is the extant slickhead, a deep-sea relative of tiny Prohalecites. Anal fin entirely behind the dorsal fin, multiserial teeth on jaws, more scale rows than congeners, precaudal vertebrae less than 30, multiserial teeth on jaws, more scale rows than congeners, precaudal vertebrae less than 30, seven branchiostegal rays, two epurals, and head smaller than those of relatives. Most slickheads are benthopelagic or mesopelagic feeders of gelatinous zooplankton, but behavioural observations and dietary analyses indicate that the new species is piscivorous.

Prohalecites porroi
Bellott 1857, Tintori 1990, MCSNIO P 348, Middle Triassic; 4cm) is a late surviving basal bony ray-fin fish (Actinopterygia, Osteichthyes). No trace of scales is preserved in any specimen (as in slickhead heads, Figs 1, 2). No neurocranial material is preserved. Tintori left Prohalecites as a Neopterygian incertae sedis, “because its characters do not perfectly fit in any of these cited groups.” Hemichordacentra are present. The preopercular is so slender it is rodlike.
Changes like this
(Fig 1) are only one reason why housekeeping on the ray-fin subset of the LRT is taking so long (moving from months to seasons).
This appears to be a novel hypothesis of interrelationships.
If not, please provide a citation so I can promote it here.
References
Alcock AW 1890. Natural history notes from H. M. Indian marine survey steamer ‘Investigator’, Commander R. F. Hoskyn, R. N., commanding. No. 18. On the bathybial fishes of the Arabian Sea, obtained during the season 1889-1890. [Unspecified Publisher] Vol. 6: 295-311.
Arratia G and Tintori A 1999. The caudal skeleton of the Triassic actinopterygian †Prohalecites and its phylogenetic position, p. 121–142. In: Mesozoic Fishes 2—Systematics and Fossil Record. G. Arratia and H.-P. Schultze (eds.). Verlag Dr. F. Pfeil, München.
Arratia G 2015. Complexities of early Teleostei and the evolution of particular morphological structures through time. Copeia 103(4):999–1025.
Bellotti C 1857. Descizione di alcune nuove specie di pesci fossili di Perledo e di altre localtta lombarde. 419–432. In Sopani A (ed) Studi geologici sulla Lomabardia. Editore Turati, Milano.
Fujiwara Y et al. 2021. Discovery of a colossal slickhead (Alepocephaliformes: Alepocephalidae): an active-swimming top predator in the deep waters of Suruga Bay,
Japan. Nature Scientific Reports 11:2490
Tintori A 1990. The actinopterygian fish Prohalecites from the Triassic of northern Italy. Palaeontology 33:155–174.