Gaillard, MacPhee and Forasiepi 2023 reported,
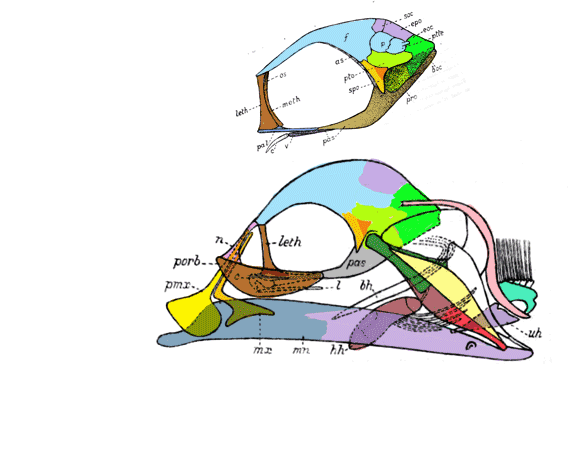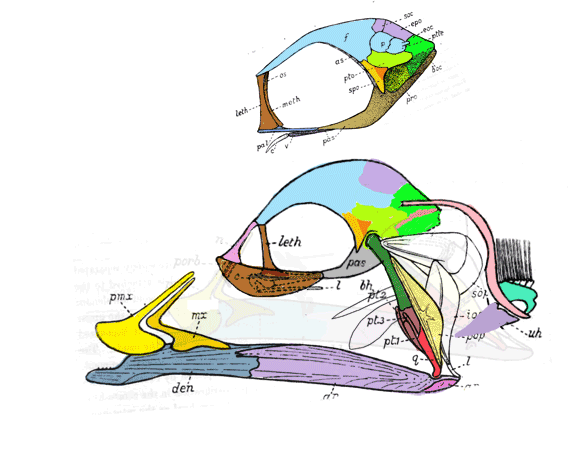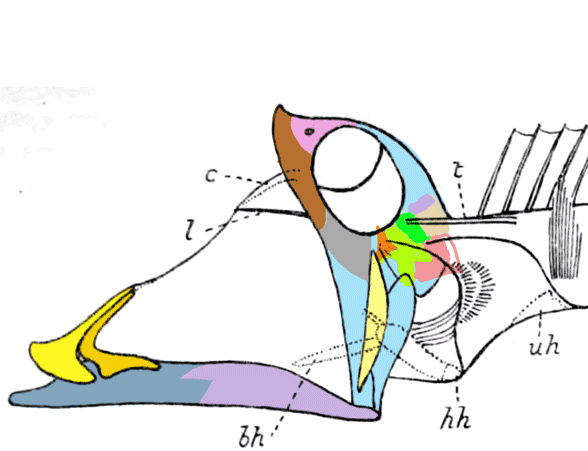
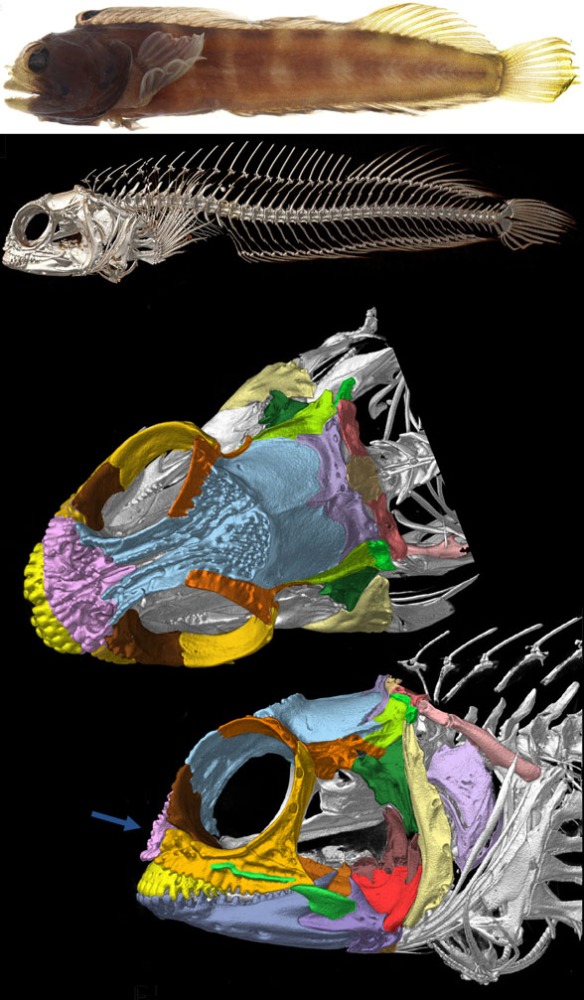
“Adaptations for good stereopsis have evolved in living predaceous mammals, and it is reasonable to infer that fossil representatives would follow the same pattern. This applies to the sparassodonts, an extinct group of South American hypercarnivores related to marsupials, with one exception. In the sabertooth Thylacosmilus atrox, (Fig 1) the bony orbits were notably divergent, like those of a cow or a horse, and thus radically differing from conditions in any other known mammalian predator.”
Not true. ‘Divergent orbits’ (Fig 1) is a trait common to clade members (e.g. Fig 4), as one should expect in any clade. Unfortunately phylogenetic analysis was not part of this study and several marsupial sabertooth clade members were ignored.
Readers, whenever you see the term, “radically differing”, assume the authors have not done their homework (or might be seeking headlines). Evolution never produces anything radically different. It always works in baby steps (= microevolution). Relatives share traits.
Sprassodonta is polyphyletic according to the large reptile tree (LRT, 2224 taxa) with some traditional members nesting in Placentalia, others in Marsupialia.

The authors’ report,
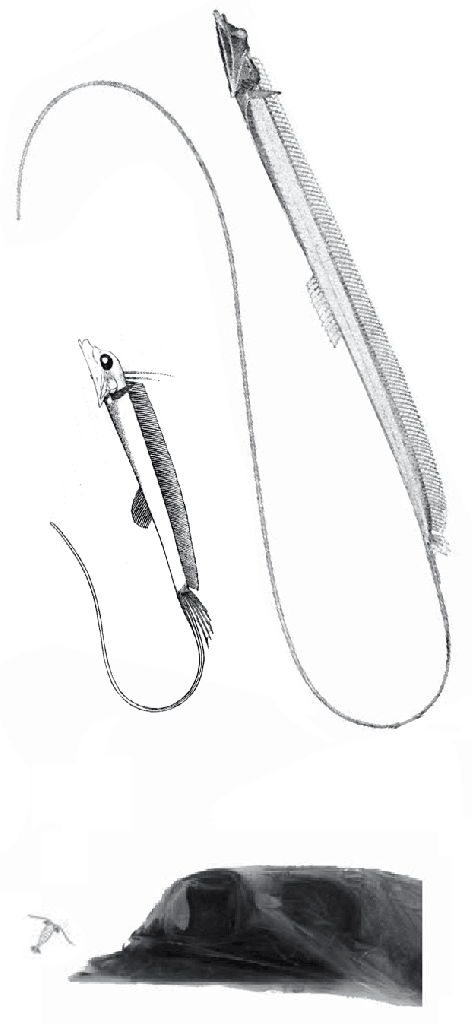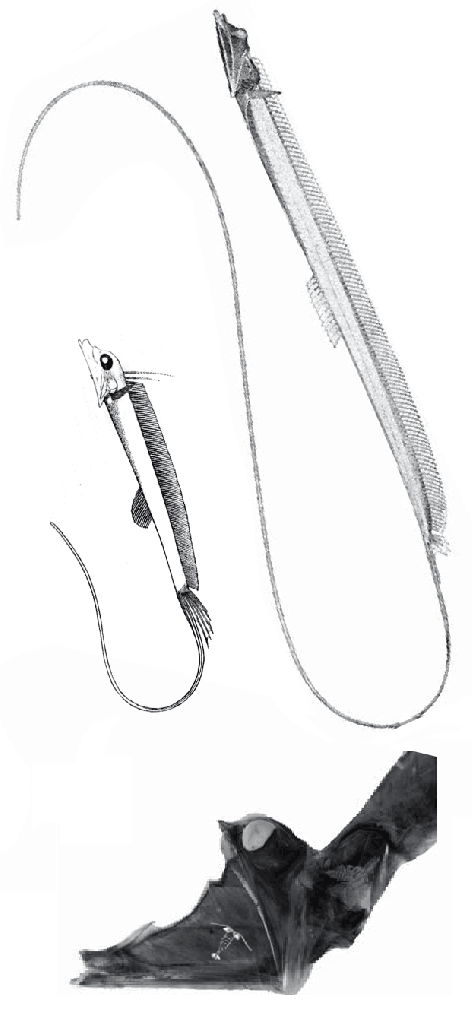
“The factors that prompted orbital reorganization in Thylacosmilus are unknown…” that may be because only one other ancestral taxon was listed in the text (house-cat sized Patagosmilus, Fig 2), but not illustrated. The authors could have answered this question by testing ancestral and related taxa. They did not do so. In the LRT this sabertooth marsupial clade goes back in time to rat-sized Early Cretaceous Vincelestes (Fig 2). Even deeper in this lineage are extant Dasycercus and Dasyuroides (Fig 6), also not mentioned in the text. The latter is known to, “kill with strong bites to the head” of its victims. Phylogenetic bracketing indicates that extant habit could help us understand the habits of the extinct related Thylacosmilus (Fig 1).
The authors > also < report,
“The forcing function behind these morphological tradeoffs was the extraordinary growth of its rootless canines, which affected skull shape in Thylacosmilus in numerous ways, including relative orbital displacement.”
So, “The factors that prompted orbital reorganization in Thylacosmilus” are known, according to the authors. This is easy to see (Fig 1). The question the authorsleave unanswered (due to taxon exclusion) is: how far back does this trait extend?

Gaillard, MacPhee and Forasiepi reported,
“We show that the orbits of Thylacosmilus were frontated and verticalized in a way that favored some degree of stereopsis and compensated for limited convergence in orbital orientation.

This can’t be the first time
this ‘limited convergence in orbital orientation’ has been noted. Or is it? Have these three authors finally unveiled the obvious? Or are they making a big deal out of something everyone is already aware of?

Barbourofelis
(Fig 3) is traditional nimravid (= placental cat), but in the LRT nests with the marsupial, Thylacosmilus (Fig 1), sharing a similar skull architecture not found in placental cats (including Nimravus Fig 5) or Smilodon, Fig 4). To force Barbourofelis to move in the LRT requires 28 additional steps to Smilodon, and 34 additional steps to Nimravus.

Phylogenetic analysis answers so many questions.
Build your own LRT so you can use this powerful tool to answer phylogenetic questions like those Gaillard, MacPhee and Forasiepi both posed and overlooked.

References
Gaillard C, MacPhee, RDE and Forasiepi AM 2023. Seeing through the eyes of the sabertooth Thylacosmilus atrox (Metatheria, Sparassodonta). Nature communications biology https://doi.org/10.1038/s42003-023-04624-5