Cui et al wrote,
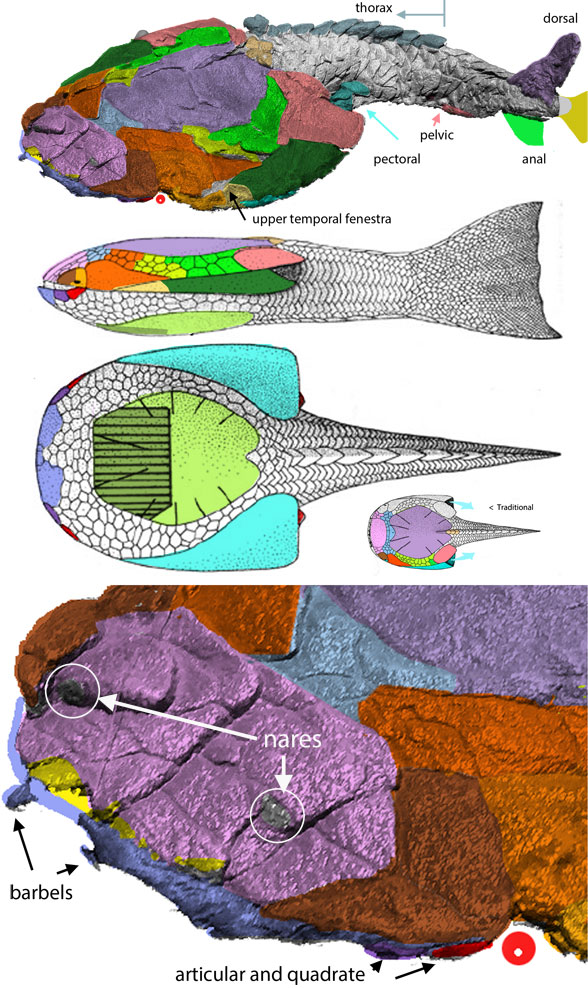
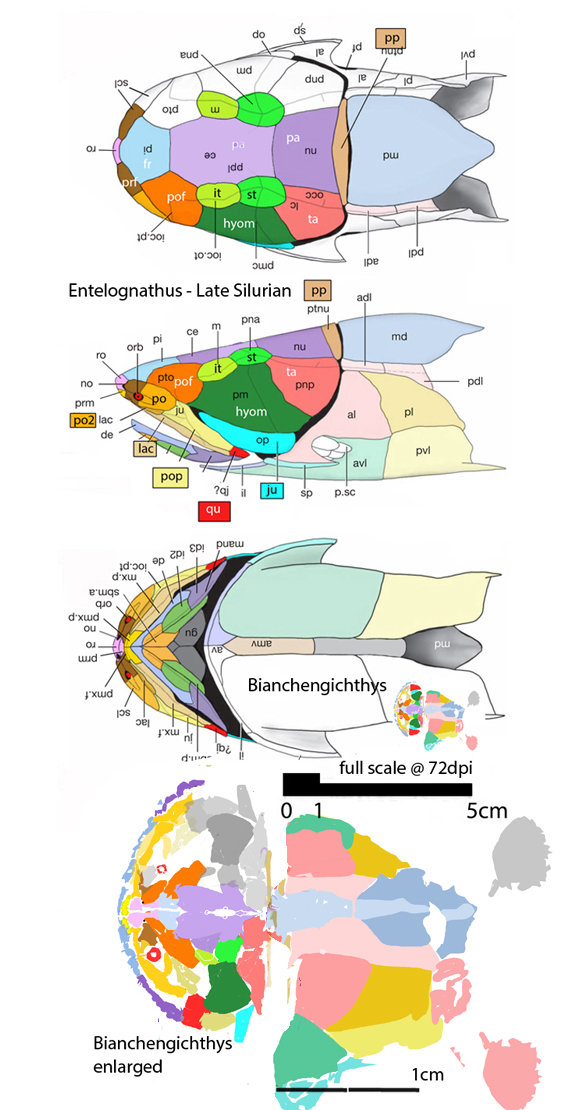
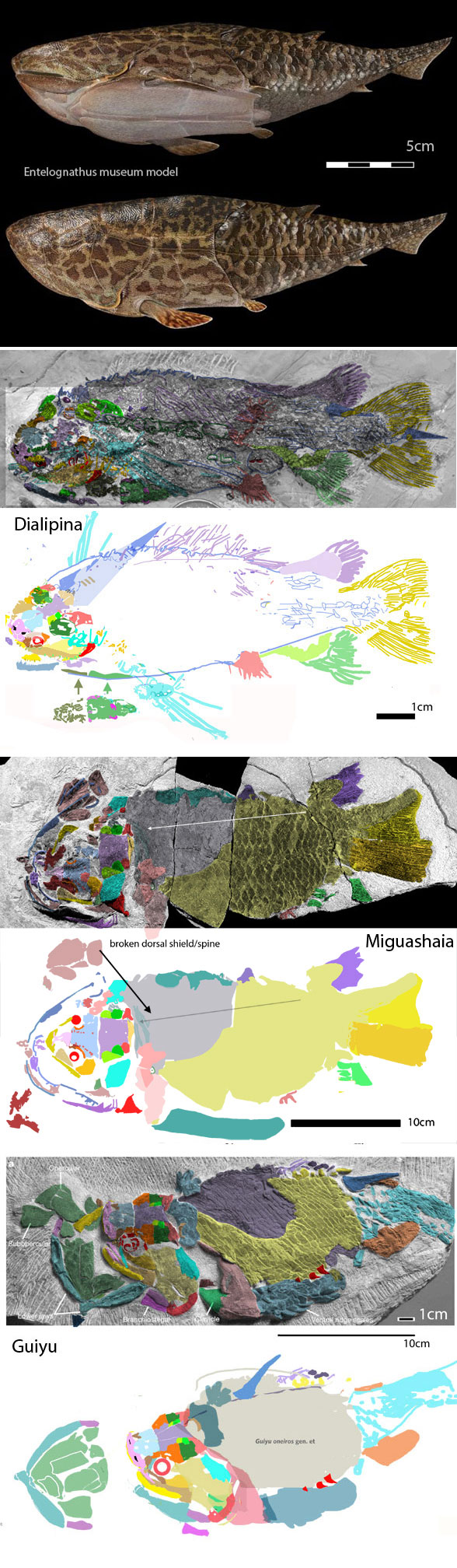
“The 425-million-year-old fish Entelognathus (Fig 1) combines an unusual mosaic of characters typically associated with jawed stem gnathostomes or crown gnathostomes. Strikingly, its scales are large and some are rhomboid, bearing distinctive peg-and-socket articulations; this combination was previously only known in osteichthyans and considered a synapomorphy of that group.”
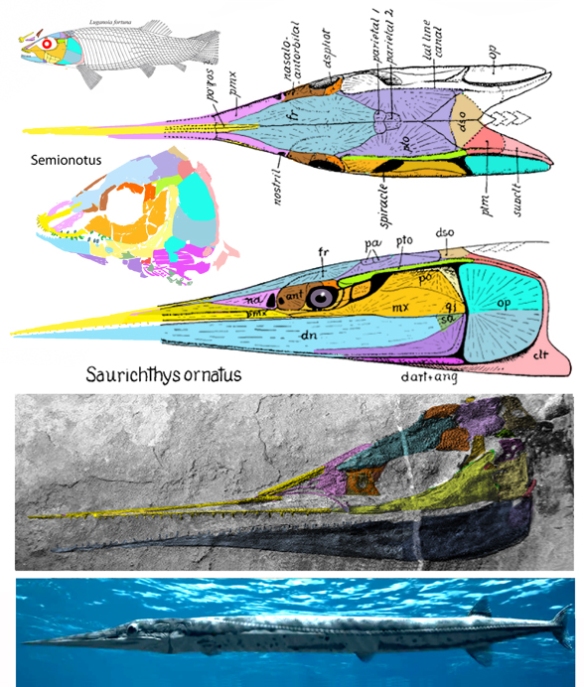
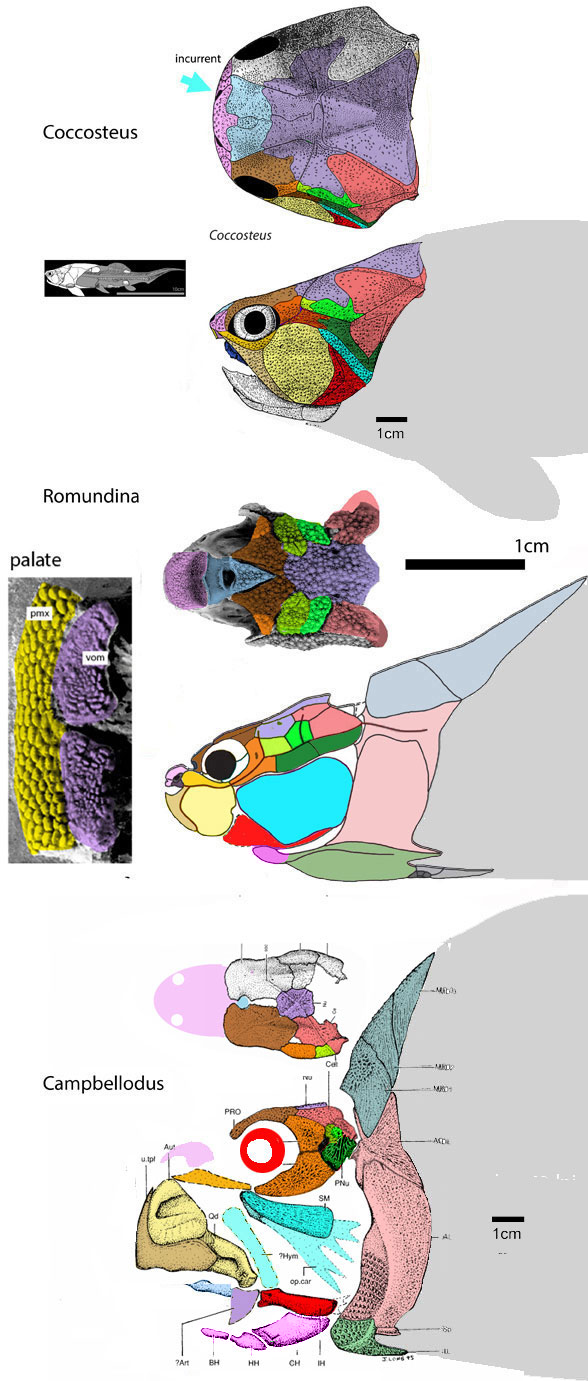
Don’t rely on traits to define a clade. We call this “Pulling a Larry Martin.” Instead use the last common ancestor method in phylogenetic analysis (Fig 2) and then describe the traits, making allowances for possible (or probable) convergence elsewhere.
Taxon exclusion appears to be responsible
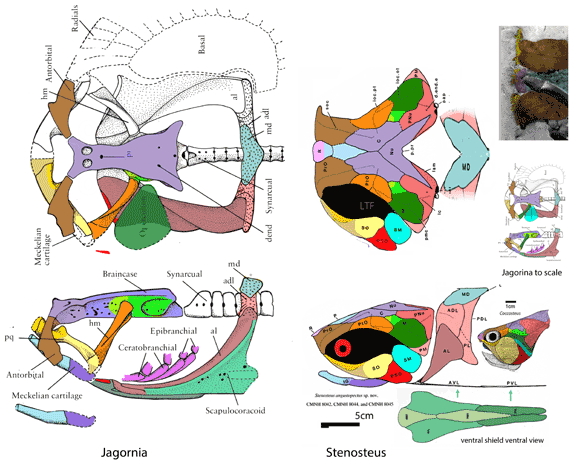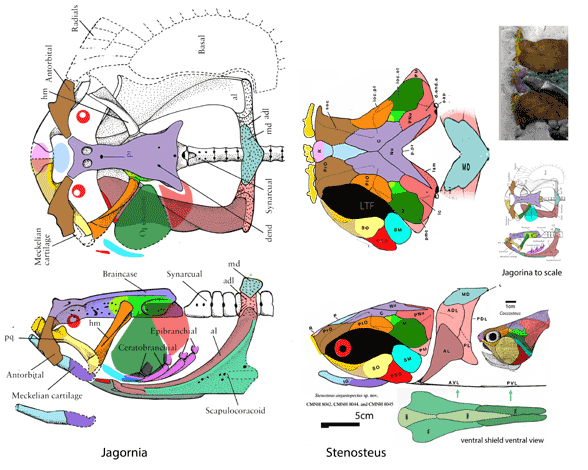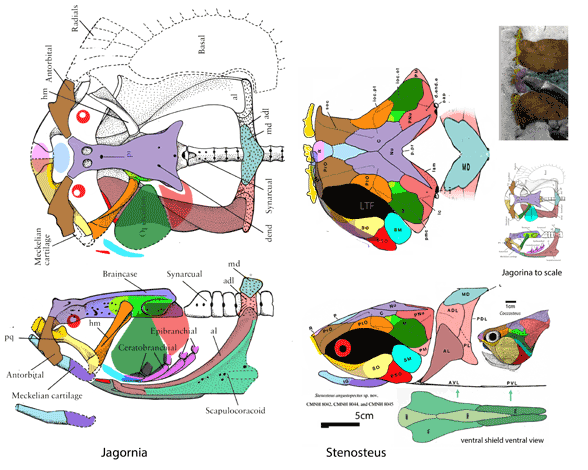
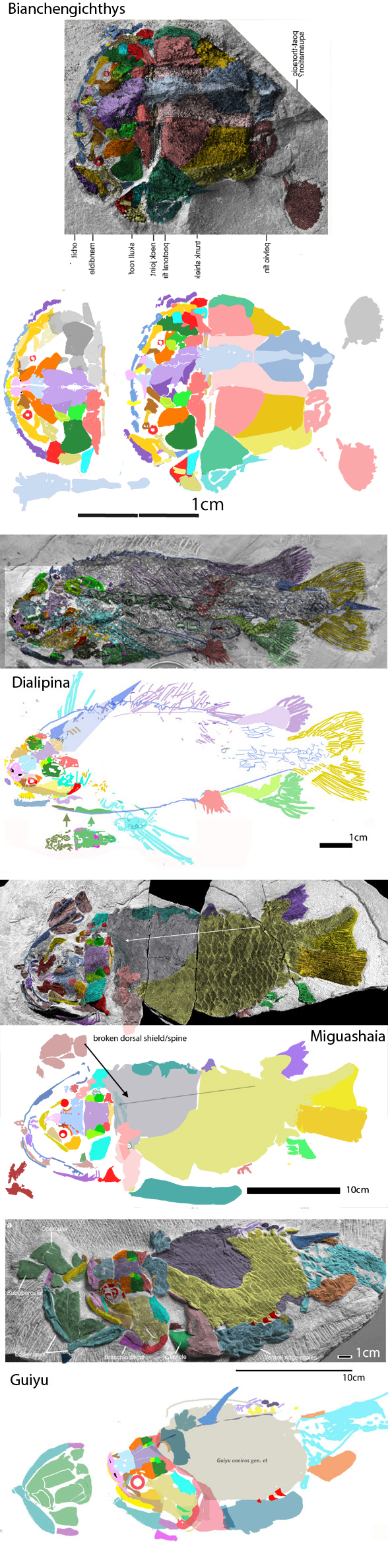
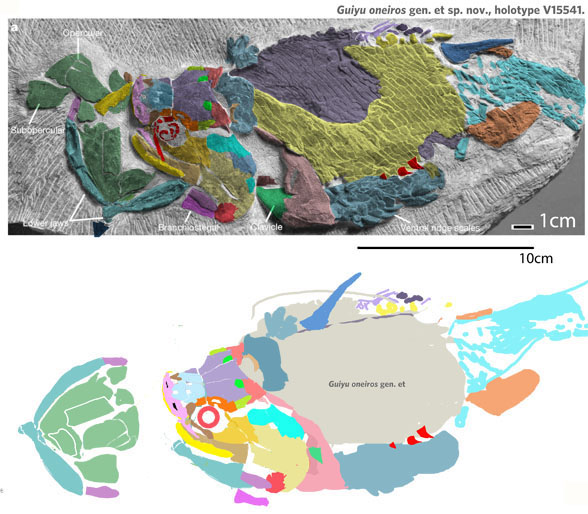
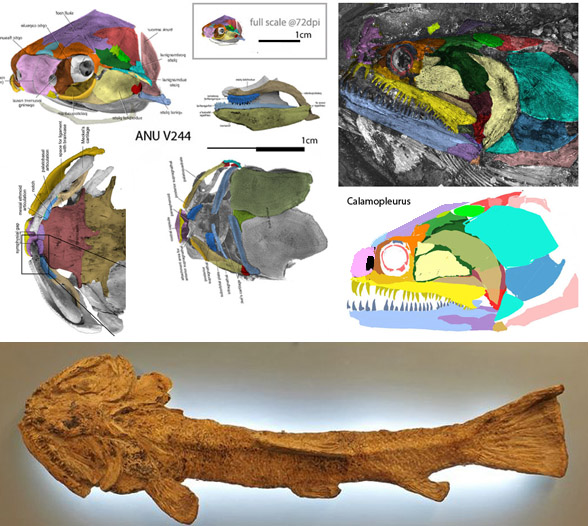
for the phylogenetic issues that vexed these authors. By testing more taxa in the large reptile tree (LRT, 2306 taxa, Fig 2) Entelognathus nested with other very similar taxa with robust scales, like Guiyu, Miguashaia and Dialipina. All four nested between traditional placoderms and traditional catfish, including Hoplosternum, the extant armored catfish. Among the three closest relatives in the LRT only Guiyu is mentioned in the Cui et al text. Catfish are not usually included in placoderm analyses.
Cui et al wrote,
“The presence in Entelognathus of an anal fin spine, previously only found in some stem chondrichthyans, further illustrates that many characters previously thought to be restricted to specific lineages within the gnathostome crown likely arose before the common ancestor of living jawed vertebrates.”
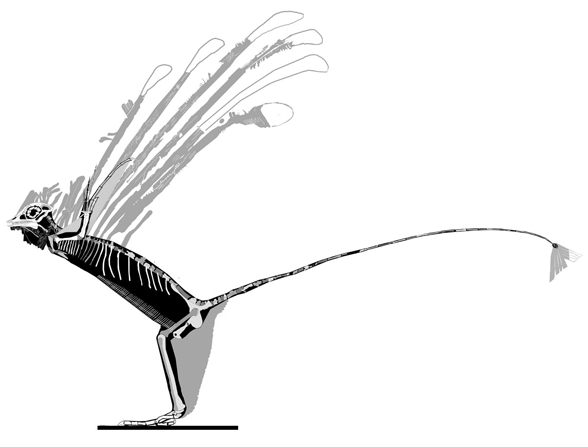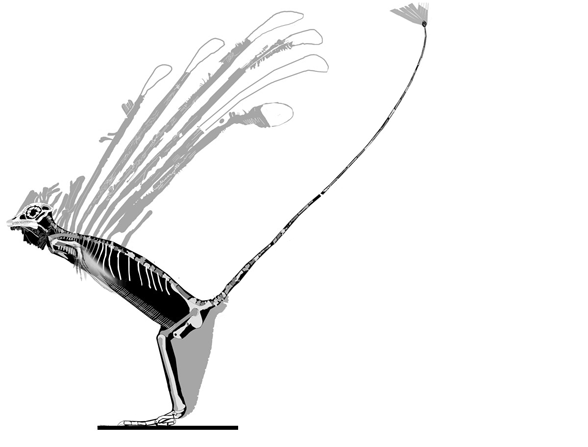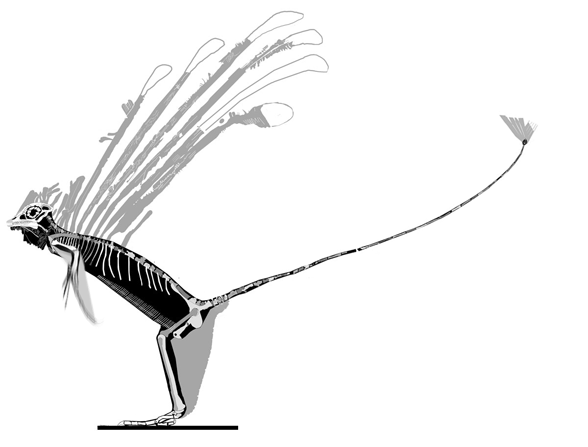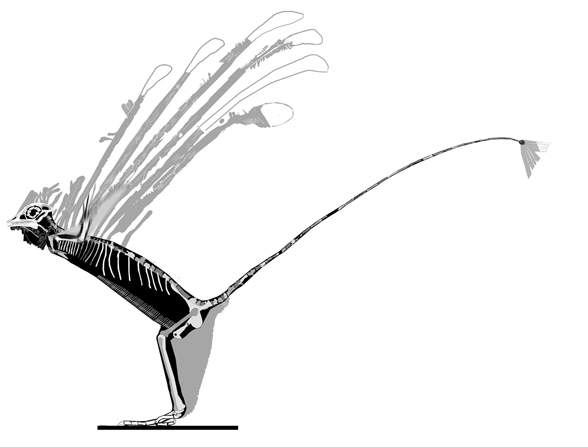
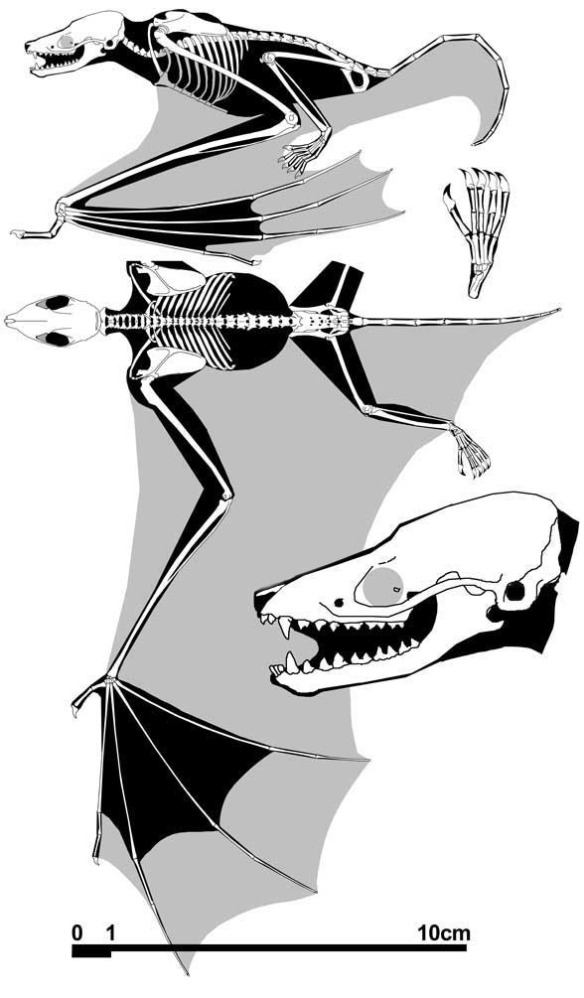
Anal fins, rather than spines, are preserved in two LRT related taxa (Fig 1). The anal portion of Guiyu is not exposed. A tiny primitive taxon, Shenacanthus, has an anal spine seen here.
The authors made the traditional mistake of assuming a single genesis of jaws in living vertebrates. The LRT falsified that in October 2023.

Cui et al wrote,
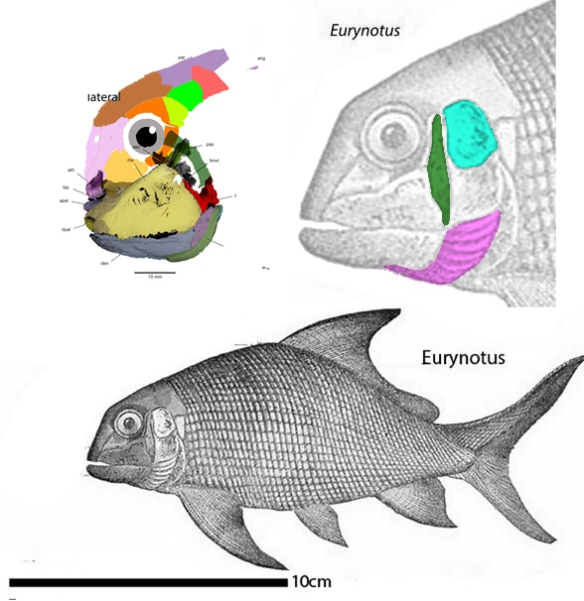
“The phylogenetic analysis placed Entelognathus and Qilinyu (Fig 3) in a clade as the immediate sister lineage of crown gnathostomes, confirming both the pivotal position and themonophyly ofmaxillate placoderms.”
By contrast, in the LRT Qiilinyu (Fig 3) nests with a more similar jawless taxon, Poraspis (Fig 3, not mentioned in the text), marking the genesis of placoderms prior to the invention of an anteriorly curved moveable mandible in this clade of gnathostomes.
Remember, our ancestors developed jaws independent of placoderms, sharks and catfish, as documented in October 2023 here. This very recent news post-dates submission of the Cui et al manuscript and figures to the editors of Nature. So no one in academia knew this then.
Cui et al reported,
“Our analysis generated 100,000 trees,” which was their ‘maximum trees in memory’ limit. When this happens, something (usually scoring), is wrong, based on 25 years of experience. Go back and review your scores and get rid of taxa based on scraps.
Entelognathus primordialis
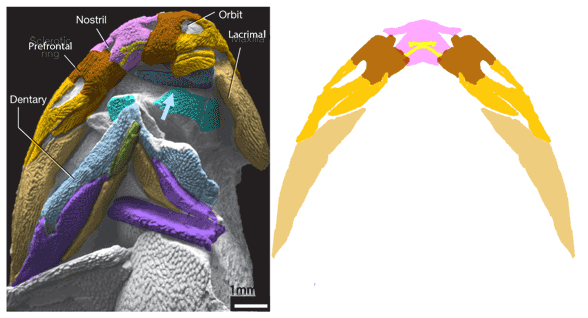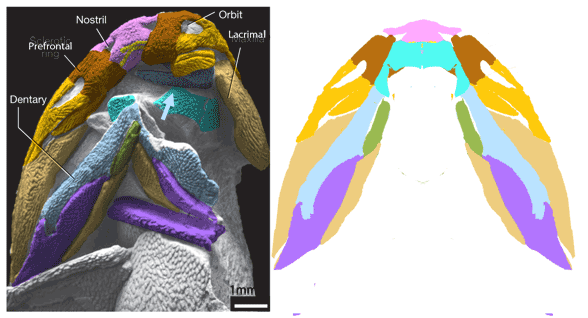
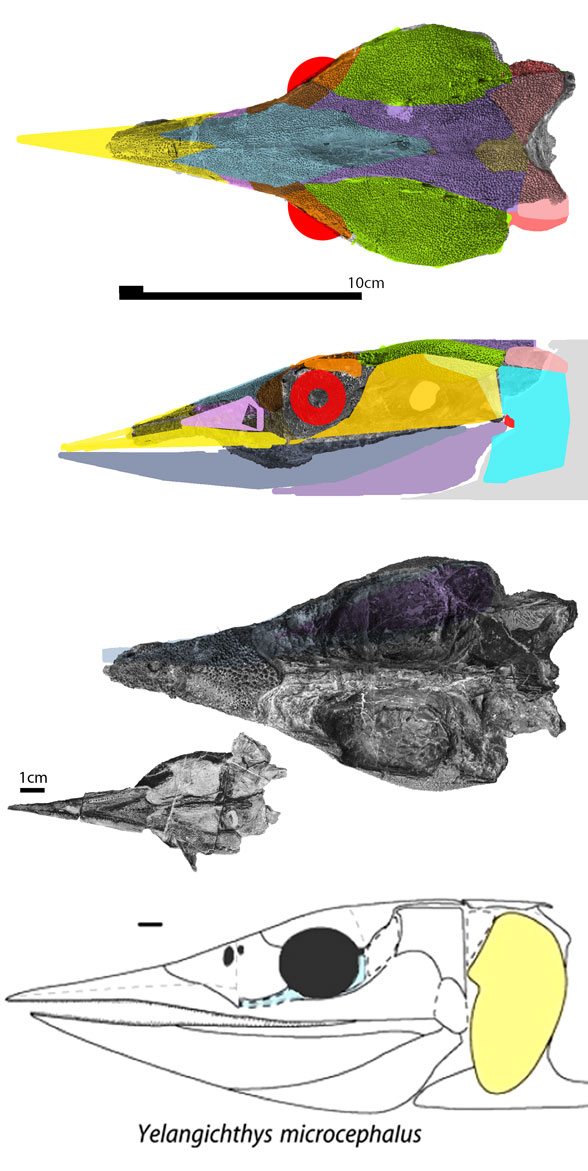
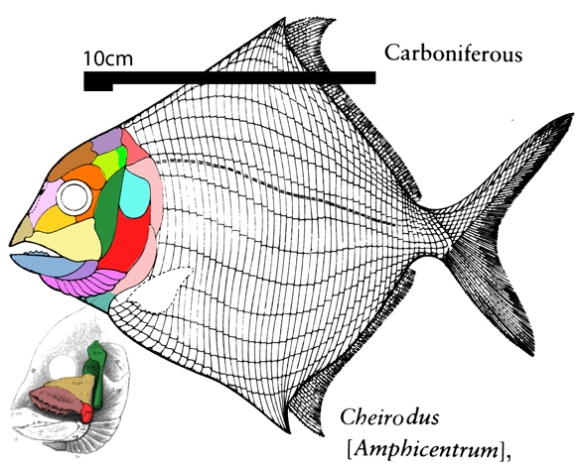
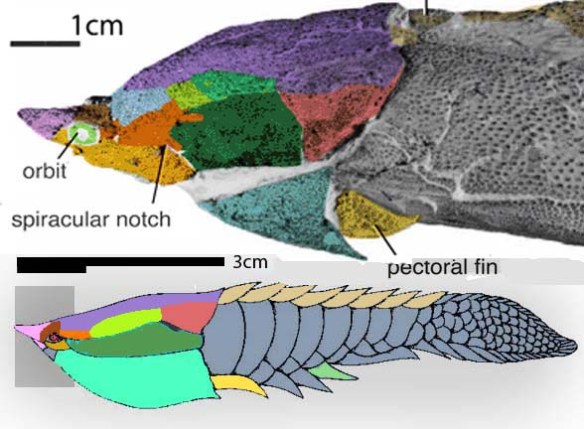
(Zhu et al. 2013; Zhu et al 2016, Cui et al 2023; Late Ludlow, Late Silurian, 419 mya; IVPP V18620) is a genus of placoderm fish with tiny eyes. Here (Fig 4) skull bones are re-identified with their tetrapod homologies. Pre-teeth are tiny pustules and wrinkles on the bone. Only a few days ago we looked at the scooping mouth of Entelognathus.
Documenting the back half of Entelognathus is welcome news.
The heavy scalation comes as no surprise to the LRT which was expecting that based on the similar traits found in related taxa (= phylogenetic bracketing, Fig 1 ). It is also important to keep working on your cladogram if it keeps recovering the maximum limit of trees (see above). Add taxa and re-check your scores. Ideally a single, fully-resolved tree should be your goal (if you can avoid using taxa based on scraps).
References
Cui X-D, Friedman M, Yu Y, Zhu Y-A and Zhu M 2023. Bony-fish-like scales in a Silurian maxillate placoderm. Nature Communications doi.org/10.1038/s41467-023-43557-9
Zhu M, Yu X-B, Ahlberg PE, Choo B and 8 others 2013. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature. 502:188–193.
Zhu M et al. 2016. A Silurian maxillate placoderm illuminates jaw evolution. Science 354.6310 (2016): 334-336.