The hyper-elongate neck developed by convergence three times
(Figs. 1, 2) in the known prehistory of marine tetrapods. On land we have giraffes, langobardisaurs and sauropods, but they are not considered here due to their separate terrestrial environs. Based on the similar necks and diets (fish and squid of these three marine tetrapods, perhaps some of the mystery surrounding these taxa can be resolved.

Figure 1. Albertonectes, Tanystropheus and Dinocephalosaurus to scale. With all the other predators assuming a horizontal pose, maybe the vertical neck of these predators in the midst of schools of fish and squid went unnoticed…until it was too late. Maybe those rocks in the belly of the elasmosaur helped keep it anchored.
The three marine taxa with hyper elongate necks
(Fig. 1) are Albertonectes (Elasmosauridae), Tanystropheus (Tritosauria), and Dinocephalosaurus (Tritosauria). We also know of several specimens closely related to each of these taxa, discussed here, here, and here. They all share more than a hyper-elongate neck in common, but that’s the one thing that predominates. They appear to have all been marine vertical predators, passively extending their neck up into schools of prey, essentially unrecognized because they were not horizontal speedsters, like all the other predators out there.

Figure 2. Skulls of Albertonectes, Dinocephalosaurus and two types of Tanystropheus skulls compared, not to scale. Lots of convergence here, it’s plain to see.
Convergent skull traits in vertical feeders:
- Small skull
- Long procumbent teeth
- Large premaxilla
- Upward facing eyes
- Dorsally displaced nares
- Rostrum wider than tall
- Internal naris migrated posteriorly
- Flat palate
Renesto 2005 along with Renesto and Saller 2018
presented evidence to show that Tanystropheus had a semi-aquatic horizontal lifestyle.
- “Tanystropheus was able to lift the body off the substrate when on land,
- Tanystropheus lacked adaptations for continuous swimming, either tail-based or limb-based,
- Tanystropheus was able to swim for by rowing with symmetrical strokes of the hind limbs.”
But remember,
Renesto and Saller mistakenly considered Tanystropheus a protorosaur and an archosauromorph. It is neither. In the large reptile tree (LRT, 1175 taxa) Tanystropheus nests with Huehuecuetzpalli and pterosaurs, all in the clade Tritosauria, a clade within Lepidosauria.
Renesto and Saller continue:
“The life style of Tanystropheus,the largest and most bizarre of all tanystropheids, remained uncertain since its discovery… In conclusion, Tanystropheus may have had lived in a shore line environment, where the elongate neck, may have been used to cach preys in shallow water by dashing at the prey propelled by hindlimbs, either starting from the shoreline from a resting positionor, in water, eventually after slowly closing the distance. In water, the long neck would have allowed Tanystropheus to conceal its real size while slowly approaching to fish or squid schools by reducing the disturbance caused by body surrounding water, avoiding to be detected by the prey’s lateral line. When close enough, Tanystropheus may have shifted to fast pursuit for the sudden propulsive final phase, with a series of rapid symmetrical strokes of the hind limbs (Fig. 6).”
Yeah, maybe…
but Renesto and Saller just said Tanystropheus was not a good swimmer. So let’s toss out that shift to fast pursuit.
Imagine a passive predator distinct from
all the other predators assuming a horizontal pose. Maybe the vertical neck of all these predators in the midst of schools of fish and squid went unnoticed by them…until it was too late. Maybe it’s as simple as that. No extant taxa can be used by analogy, so we have to look at extinct taxa with similar traits. We looked at Tanystropheus among the crinoids (Fig. 1), and the evolution to that niche earlier here. The convergent Dinocephalosaurus neck strike hypothesis is from Peters, Demes and Krause 2005. The long-necked limbed ancestors of elasmosaurs were morphologically similar and coeval to long-necked limbed tritosaurs in the Middle Triassic (Fig. 5).
Note added on the vertical neck:
Peters, Demes and Krause 2005 (actually just Peters, in this case, as there were three comments to the original Dinocephalosaurus paper (Li, Rieppel and LaBarbera 2004), now lumped into one reply) suggested that breathing would have been difficult for long-necked underwater taxa due to changes in pressure with increasing depth, but these taxa could swallow air at the surface then lower the neck to allow the air bubble to rise into the lungs. Just a few degrees of declination would do the trick.
Renesto and Saller report:
“The study focused mostly on the post-dorsal sections of the vertebral column, on the pelvis and hind limb.” Ignoring the neck in Tanystropheus ignores the biggest clue to its niche. Let’s not do that.

Figure 3. The Middle Triassic world with the tropical shallow San Giorgio area where Tanystropheus is found highlighted. Warm waters enabled Tanystropheus and other Triassic reptiles to stay submerged continually.
Another dinocephalosaur
was reconstructed here (Fig. 3). The neck in this specimen is so gracile, it is difficult to imagine it in any active mode, so the vertical passive pose remains as the only viable alternative. These are not active predators.
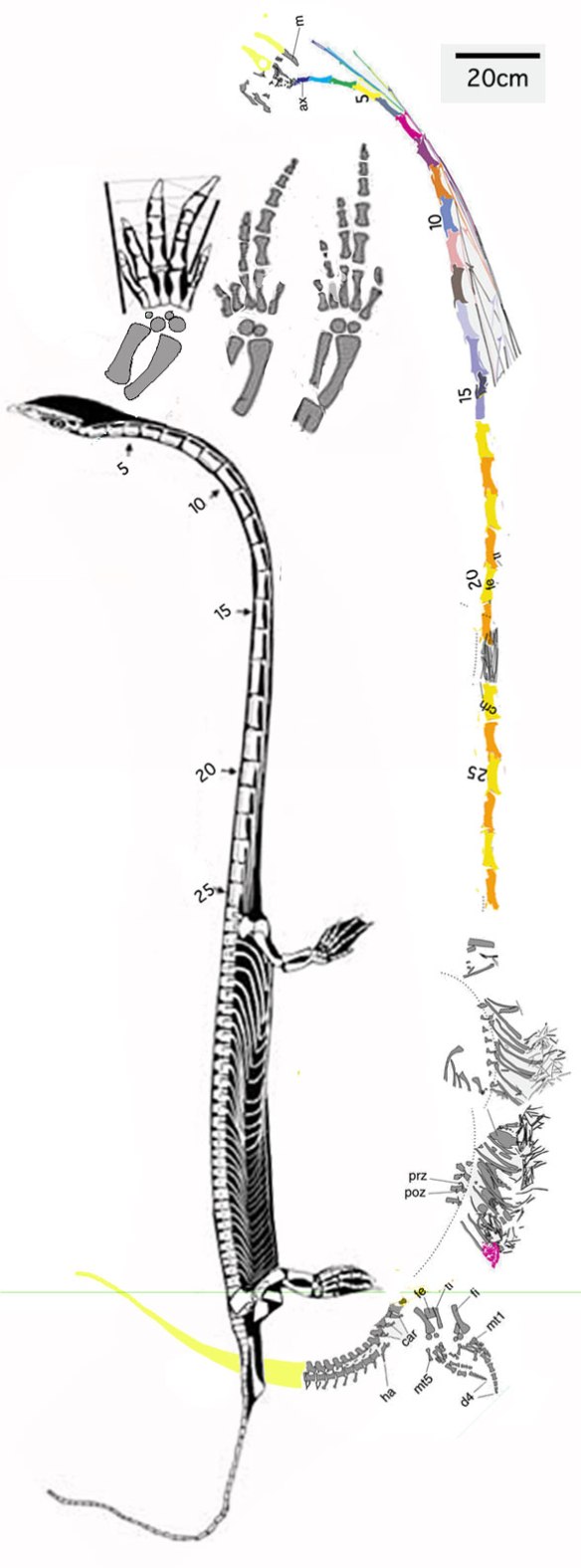
Figure 4. The new Dinocephalosaurus has traits the holotype does not, like a longer neck with more vertebrae, a robust tail with deep chevrons and a distinct foot morphology with an elongate pedal digit 4.
And finally
Note the long neck of the derived nothosaur/pre-elasmosaur, like Wangosaurus (Middle Triassic), preceded the evolution to flippers found in later vertical feeding elasmosaurs (Fig. 5). Like the the coeval vertical predators tanystropheids (Fig. 1) and dinocephalosaurs (Fig. 4) pre-elasmosaurs like Wangosaurus out-competed these similar tritosaur lepidosaurs, which cannot be found after the Triassic. Clearly pre-elasmosaurs were not such great swimmers when they started, and must have been only marginally better swimmers after their small limbs became small flippers. Given this data, the hypothesis of vertical predation of small squid and fish prey in pre-elasmosaurs and their elasmosaur descendants deserves the opportunity to be falsified.
By contrast, pliosaurs,
like Brachauchenius, with their big flippers and large toothy skulls, were excellent horizontal predators and fast swimmers. This contrast is key to the present hypothesis.

Figure 5. Elasmosaurid origins. The long neck preceded the flippers in this clade of vertical feeders.
Additional data:
Albertonectes vanderveldei (Kubo et al. 2012; Upper Campanian, Alberta; TMP 2007.022.0002) is a virtually complete elasmosaur 11.2m in length (the longest of any elasmosaur) lacking only the skull. It had a 7m neck of 76 vertebrae, the most of any vertebrate. Stones in the belly might have kept it anchored. The gizzard in birds is located posteriorly, as seen in this elasmosaur.
Tanystropheus longobardicus (Tanystropheus conspicuus von Meyer 1855, Tribelesodon longobardicus Bassani 1886, Tanystropheus longobardicus Peyer 1930) Anisian, Middle Triassic, ~240 mya, ~4.5m in length, was considered a pterosaur before Peyer (1930) established that the long bones were neck bones, not wing bones. Derived from a sister to the the T4822 specimen of Macrocnemus, Tanystropheus was a sister to the much smaller Tanytrachelos and Langobardisaurus, rather than the convergent Dinocephalosaurus. Warm waters enabled Tanystropheus and other Alpine Triassic reptiles to stay submerged continually.
Dinocephalosaurus orientalis (Li, Rieppel and LaBarbera 2004) Late Ladinian, Middle Triassic ~228 mya, was orginally considered a marine sister to Tanystropheus with limbs nearly transformed into paddles of similar size. Phylogenetic analysis places it closer to a specimen of Macrocemus, T2472. Dinocephalosaurus was not a protorosaur, as originally described. Rather Dinocephalosaurus was a tritosaur lepidosaur The skull was described as crushed, but it was actually quite flat in life with dorsally directed orbits. The ribs were also much wider than deep. Both of these are characters found in bottom dwellers, not free-swimmers. The cervical (25) and dorsal (33) counts are the highest among tanystropheids. The limbs were short but the hands and feet were relatively large, paddle-like and probably webbed.

Figure 6. A squad of squid, food for both tanystropheids and elasmosaurs.
References
Bassani F 1886. Sui Fossili e sull’ età degli schisti bituminosi triasici di Besano in Lombardia. Atti della Società Italiana di Scienze Naturali 19:15–72.
Li C, Rieppel O and LaBarbera MC 2004. A Triassic aquatic protorosaur with an extremely long neck. Science 305:1931.
Liu, J. et al. 2017. Live birth in an archosauromorph reptile. Nature Communications 8, 14445 doi: 10.1038/ncomms14445
Peters D, Demes B and Krause DW 2005. Suction feeding in Triassic Protorosaur? Science, 308: 1112-1113.
Kubo T, Mitchell MT and Henderson DM 2012. Albertonectes vanderveldei, a new elasmosaur (Reptilia, Sauropterygia) from the Upper Cretaceous of Alberta. Journal of Vertebrate Paleontology 32 (3): 557-572. DOI:10.1080/02724634.2012.658124.
Li C 2007. A juvenile Tanystropheus sp.(Protoro sauria: Tanystropheidae) from the Middle Triassic of Guizhou, China. Vertebrata PalAsiatica 45(1): 37-42.
Li C, Rieppel O and LaBarbera MC 2004. A Triassic aquatic protorosaur with an extremely long neck. Science 305:1931.
Liu, J. et al. 2017. Live birth in an archosauromorph reptile. Nature Communications 8, 14445 doi: 10.1038/ncomms14445
Lockley MG 2006. Observations on the ichnogenus Gwineddichnium and wyneddichnium-like footprints and trackways from the Upper Triassic of the Western United States. In: Harris JD, Lucas SG, Spielmann JA, Lockley MG, Milner ARG. & Kirkland JI (Eds) – The Triassic-Jurassic Terrestrial Transition. New Mexico Museum of Natural History Science Bulletin 37: 170-175.
Meyer H von 1847–55. Die saurier des Muschelkalkes mit rücksicht auf die saurier aus Buntem Sanstein und Keuper; pp. 1-167 in Zur fauna der Vorwelt, zweite Abteilung. Frankfurt.
Nosotti S 2007. Tanystropheus longobardicus (Reptilia, Protorosauria: Reinterpretations of the anatomy based on new specimens from the Middle Triassic of Besano (Lombardy, Northern Italy). Memorie della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano, Vol. XXXV – Fascicolo III, pp. 1-88
Peters D, Demes B and Krause DW 2005. Suction feeding in Triassic Protorosaur? Science, 308: 1112-1113.
Peyer B 1931. Tanystropheus longobardicus Bass sp. Die Triasfauna der Tessiner Kalkalpen. Abhandlungen Schweizerische Paläontologie Gesellschaft 50:5-110.
Renesto S 2005. A new specimen Tanystropheus (Reptilia Protorosauria) from the Middle Triassic of Switzerland and the ecology of the genus: Rivista Italiana di Paleontologia e Stratigrafia 111(3): 377-394.
Renesto S and Saller F 2018. Evidences for a semi aquatic life style in the Triassic diapsid reptile Tanystropheus. Rivista Italiana di Paleontologia e Stratigrafia 124(1):23-34.
Rieppel O, Jiang D-Y, Fraser NC, Hao W-C, Motani R, Sun Y-L & Sun ZY 2010. Tanystropheus cf. T. longobardicus from the early Late Triassic of Guizhou Province, southwestern China. Journal of Vertebrate Paleontology 30(4):1082-1089.
Wild R 1973. Die Triasfauna der Tessiner Kalkalpen XXIII. Tanystropheus longobardicus(Bassani) (Neue Ergebnisse). – Schweizerische Paläontologische Abhandlungen 95: 1-162 plus plates.
wiki/Dinocephalosaurus
wiki/Tanystropheus
https://en.wikipedia.org/wiki/Albertonectes







































